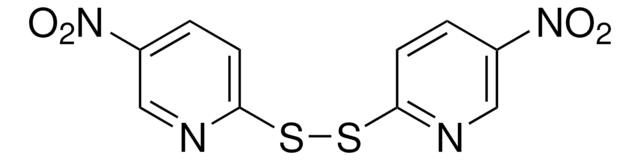
推荐产品
化驗
95%
mp
205 °C (dec.) (lit.)
溶解度
dichloromethane: soluble(lit.)
官能基
nitro
儲存溫度
2-8°C
SMILES 字串
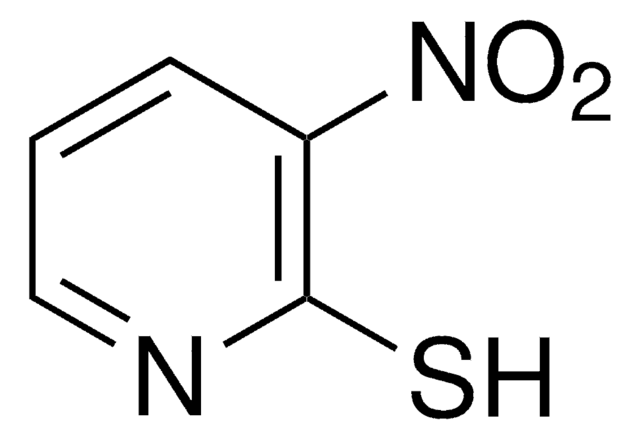
[O-][N+](=O)c1cccnc1SCl
InChI
1S/C5H3ClN2O2S/c6-11-5-4(8(9)10)2-1-3-7-5/h1-3H
InChI 密鑰
WTKQMHWYSBWUBE-UHFFFAOYSA-N
一般說明
3-硝基-2-吡啶亚磺酰基苯基(Npys)部分可用作半胱氨酸的保护活化基团,特别是在环状和不对称二硫化物的合成中。研究了 NpysCl 在各种溶剂中的稳定性。
應用
使用 3-硝基-2-吡啶亚磺酰氯(NpysCl)作为合成 N-, O-和 S-Npys-保护的氨基酸的原料。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
K C Pugh et al.
International journal of peptide and protein research, 42(2), 159-164 (1993-08-01)
3-Nitro-2-pyridinesulfenyl chloride (NpysCl) is the starting material for the synthesis of N-, O- and S-Npys-protected amino acids. Two efficient, novel synthetic routes to NpysCl are described. The stability of NpysCl was determined in a variety of solvents, with and without
Y Shimohigashi et al.
Journal of chromatography, 597(1-2), 425-428 (1992-04-24)
The thiol groups of leucinthiol, cysteamine and cysteine incorporated into opioid peptides enkephalin and morphiceptin were activated by the 3-nitro-2-pyridinesulphenyl (Npys) group to form mixed disulphides highly reactive to a free thiol. Enkephalin analogues containing Npys-leucinthiol or -cysteine at positions
Alisson L Matsuo et al.
Biochemical and biophysical research communications, 355(4), 1000-1005 (2007-03-03)
The inhibitory capacity of C-Npys (S-[3-nitro-2-pyridinesulfenyl]) derivatives over thiol-containing serine proteases has never been tested. In the present work we used an extracellular serine-thiol proteinase activity from the fungal pathogen Paracoccidioides brasiliensis (PbST) to describe a potent inhibitory capacity of
R Matsueda et al.
Peptide research, 5(5), 262-264 (1992-09-01)
Two recent reports on the partial lability of the 3-nitro-2-pyridinesulfenyl (Npys) thiol protecting group towards 1-hydroxy-benzotriazole (HOBt) have prompted a rechecking of the chemical behavior of this group. Using both soluble and polymer-bound forms of Cys(Npys) as test materials, the
R G Simmonds et al.
International journal of peptide and protein research, 43(4), 363-366 (1994-04-01)
The 3-nitro-2-pyridinesulphenyl (Npys) moiety is finding increasing utility as a protecting-activating group for cysteine, particularly in the synthesis of cyclic and unsymmetrical disulfides using the Boc strategy. This chemistry has been extended to peptides assembled by the Fmoc strategy. N-Terminal
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门