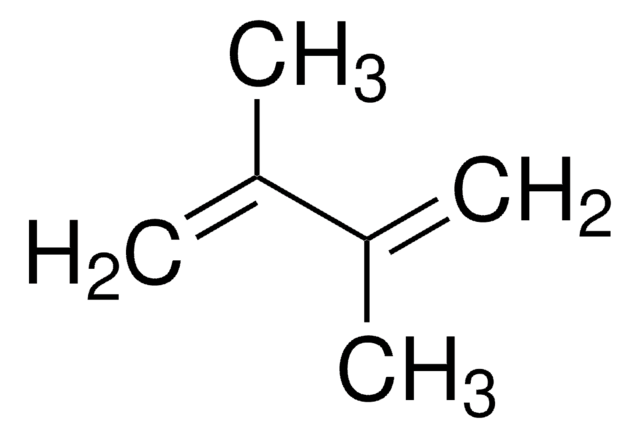
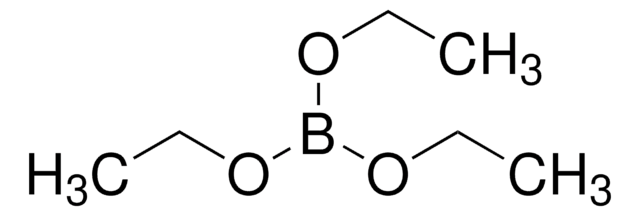
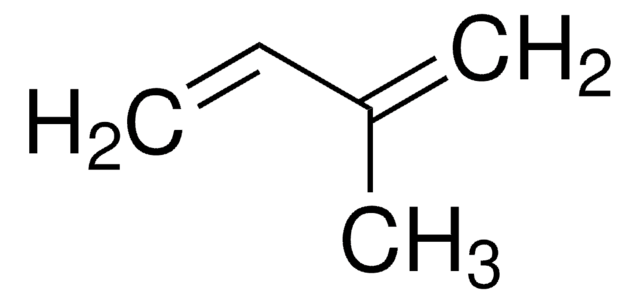
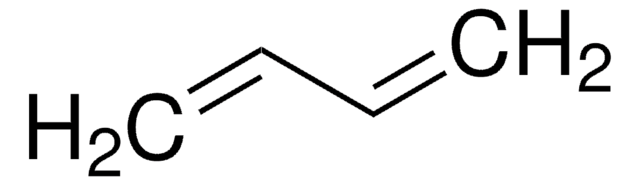
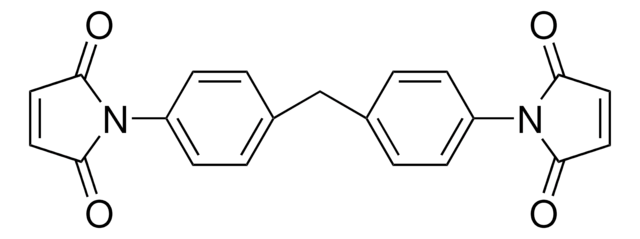
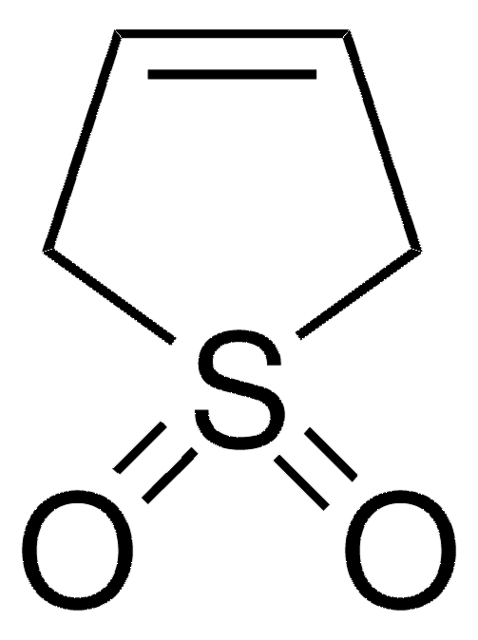
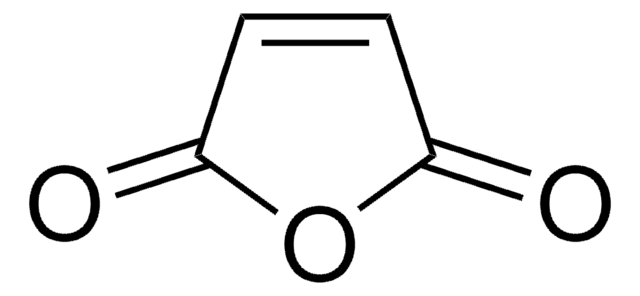
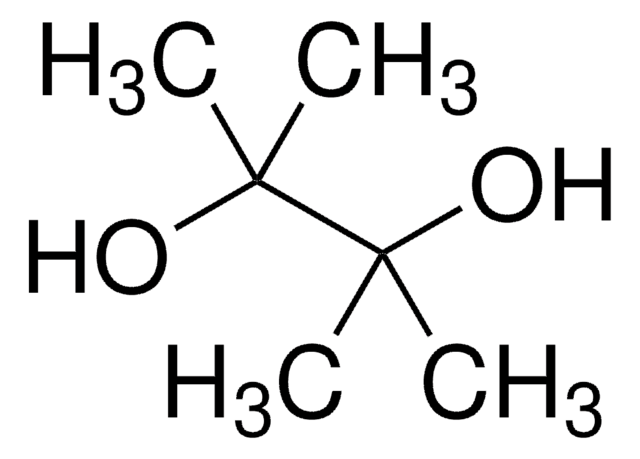
推荐产品
蒸汽密度
1.9 (15 °C, vs air)
品質等級
蒸汽壓力
1863 mmHg ( 21 °C)
化驗
≥99%
自燃溫度
788 °F
包含
p-tert-butylcatechol as inhibitor
expl. lim.
12 %
bp
−4.5 °C (lit.)
mp
−109 °C (lit.)
溶解度
water: soluble 0.5 g/L at 20 °C
密度
0.62 g/mL at 20 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
C=CC=C
InChI
1S/C4H6/c1-3-4-2/h3-4H,1-2H2
InChI 密鑰
KAKZBPTYRLMSJV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
It may be used in the synthesis of the following:
- 1-Silyl-substituted 1,3-butadienes, by [RuHCl(CO)(PCy3)2]-catalyzed silylative coupling of terminal (E)-1,3-dienes with vinylsilanes.
- Synthetic rubber and thermoplastic resins.
- Disilylated dimers by reacting with chlorosilanes.
- Octa-2,7-dien-1-ol via palladium catalyzed-hydrodimerization.
生化/生理作用
包裝
Compatible with the following:
法律資訊
也與該產品經常一起購買
軟管倒鉤
訊號詞
Danger
危險聲明
危險分類
Flam. Gas 1A - Muta. 1B - Press. Gas Liquefied gas
儲存類別代碼
2A - Gases
水污染物質分類(WGK)
WGK 3
閃點(°F)
-104.8 °F - closed cup
閃點(°C)
-76 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
商品
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
实验方案
2-Butene; 2-Methylbutane; 1,3-Butadiene; Propyne
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门