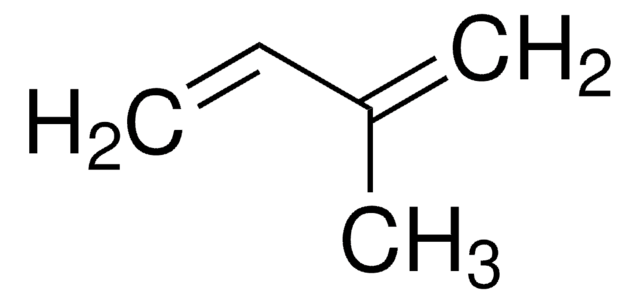
推荐产品
蒸汽壓力
269 mmHg ( 37.7 °C)
品質等級
化驗
98%
形狀
liquid
包含
100 ppm BHT as stabilizer
折射率
n20/D 1.438 (lit.)
bp
68-69 °C (lit.)
mp
−76 °C (lit.)
密度
0.726 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
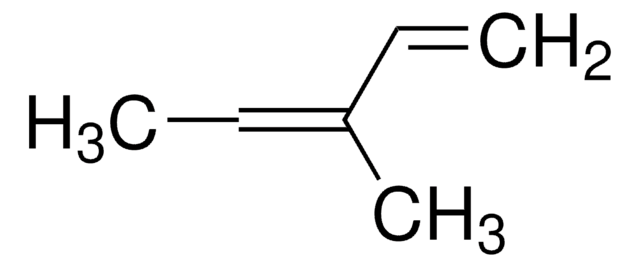
SMILES 字串
CC(=C)C(C)=C
InChI
1S/C6H10/c1-5(2)6(3)4/h1,3H2,2,4H3
InChI 密鑰
SDJHPPZKZZWAKF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
它可用于以下过程:
- 在钴催化剂存在下通过 1,4-氢化丁二烯基化制备相应的的 1-芳基取代 1,3-二烯的 1,3,6-三烯衍生物。
- 与 gem-氰基硝基乙烯反应合成 6-芳基(杂环芳基)-3,4-二甲基-1-硝基-1-氰基-3-环己烯。
- 在研究 2,5-二苯基特鲁洛芬的二溴加合物的光解反应过程中,作为卤素捕集器。
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
30.2 °F - closed cup
閃點(°C)
-1 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
商品
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门