所有图片(1)
About This Item
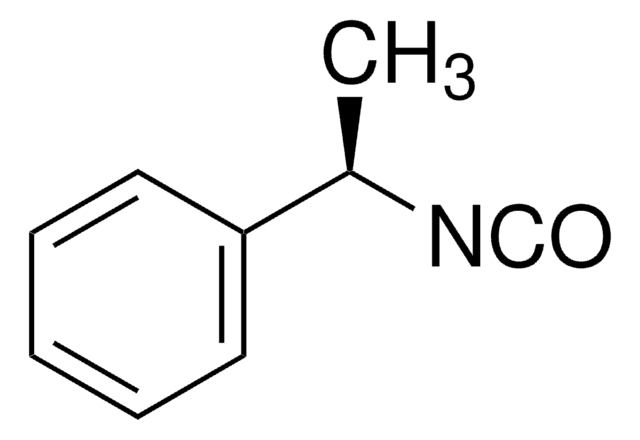
线性分子式:
C6H5CH(CH3)NCO
CAS号:
分子量:
147.17
Beilstein:
4230972
EC號碼:
MDL號碼:
分類程式碼代碼:
12352118
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
98%
形狀
liquid
光學活性
[α]20/D −10°, neat
光學純度
ee: 96% (GLC)
折射率
n20/D 1.5145 (lit.)
bp
55-56 °C/2.5 mmHg (lit.)
密度
1.045 g/mL at 20 °C (lit.)
官能基
amine
isocyanate
phenyl
儲存溫度
2-8°C
SMILES 字串
C[C@H](N=C=O)c1ccccc1
InChI
1S/C9H9NO/c1-8(10-7-11)9-5-3-2-4-6-9/h2-6,8H,1H3/t8-/m0/s1
InChI 密鑰
JJSCUXAFAJEQGB-QMMMGPOBSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
(S)-(-)-α-甲基苄基异氰酸酯是拆分对映体常用的手性衍生剂。
應用
(S)-(-)-α-甲基苄基异氰酸酯可作为拆分外消旋 (±)-6aR,11aR-高丽槐素的手性辅助剂,得到 (-)-形式,这是合成 (-)-卡本格林 A-I 的关键中间体。
訊號詞
Danger
危險分類
Acute Tox. 2 Inhalation - Aquatic Chronic 3 - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
149.0 °F - closed cup
閃點(°C)
65 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Resolution of salbutamol enantiomers in human urine by reversed-phase high performance liquid chromatography after derivatization with (S)-(-)-a-methylbenzyl isocyanate.
Kim KH, et al.
Archives of Pharmacal Research, 20(5), 486-490 (1997)
Absolute configuration and total synthesis of (-)-cabenegrin AI.
Tokes AL, et al.
Tetrahedron, 55(30), 9283-9296 (1999)
Determination of pindolol enantiomers in human plasma and urine by simple liquid-liquid extraction and high-performance liquid chromatography.
Beal JL and Tett SE
Journal of Chromatography. B, Biomedical Applications, 715(2), 409-415 (1998)
Determination of the optical purity of (R)-terbutaline by 1 H-NMR and RP-LC using chiral derivatizing agent,(S)-(-)-a-methylbenzyl isocyanate.
Kim KH, et al.
Journal of Pharmaceutical and Biomedical Analysis, 25(5), 947-956 (2001)
Myriam Matoga et al.
Journal of enzyme inhibition and medicinal chemistry, 17(6), 375-379 (2003-04-10)
The derivatization of racemic 5-[(2-methylphenoxy)methyl]-2-amino-2-oxazoline, developed as an imidazoline binding sites ligand, with (+)-(R)-alpha-methylbenzyl isocyanate was performed in chloroform. The reaction led to two pairs of diastereomers, which were separated by RP-HPLC. A kinetic study of the derivatization reaction was
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门