推荐产品
蒸汽壓力
0.5 mmHg ( 20 °C)
品質等級
化驗
98%
光學活性
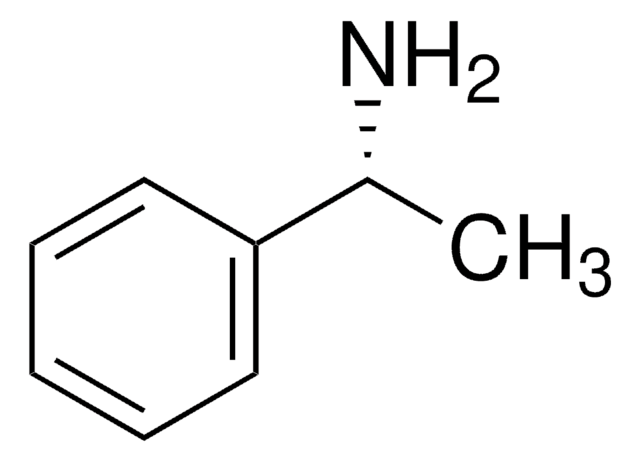
[α]20/D −39°, neat
光學純度
ee: 98% (GLC)
折射率
n20/D 1.526 (lit.)
bp
187 °C (lit.)
密度
0.94 g/mL at 25 °C (lit.)
官能基
amine
phenyl
儲存溫度
2-8°C
SMILES 字串
C[C@H](N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3/t7-/m0/s1
InChI 密鑰
RQEUFEKYXDPUSK-ZETCQYMHSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(S)-(-)-α-甲基苄胺与2-甲酰基苯基硼酸可一起用于衍生化实验方案以分析手性二醇的对映体过量值。
它也可以用于:
它也可以用于:
- 非对映选择性合成 S-氨基腈。
- 作为合成(S)-(-)-N-乙酰萼卷豆碱或(R)-(+)-N-乙酰萼卷豆碱的手性助剂。
- 作为1-取代四氢-β-咔啉不对称合成的手性合成砌块。
用于一锅多组分合成高度取代的手性吡咯。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Skin Corr. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
158.0 °F - closed cup
閃點(°C)
70 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
从最新的版本中选择一种:
分析证书(COA)
Lot/Batch Number
Ashwin R Bharadwaj et al.
Organic letters, 6(14), 2465-2468 (2004-07-02)
[reaction: see text] A multicomponent synthesis of highly substituted pyrroles catalyzed by thiazolium salts has been disclosed. The reaction employs an acyl anion conjugate addition reaction of acylsilanes (sila-Stetter) and unsaturated ketones to generate 1,4-dicarbonyl compounds in situ. The subsequent
(S)-(-)-a-Methylbenzylamine as an efficient chiral auxiliary in enantiodivergent synthesis of both enantiomers of N-acetylcalycotomine.
Ziolkowski M, et al
Tetrahedron Asymmetry, 10(17), 3371-3380 (1999)
Lithium perchlorate/diethylether catalyzed aminocyanation of aldehydes
Heydari A, et al
Tetrahedron Letters, 39(19), 3049-3050 (1998)
Alejandra León et al.
Journal of natural products, 75(5), 859-864 (2012-05-12)
The enantiomeric lactams (-)-8, (+)-8, (+)-9, and (-)-9 were formed by the reaction of the dimeric phthalide rac-tokinolide B (rac-3) with (R)-(+)-α-methylbenzylamine and (S)-(-)-α-methylbenzylamine. The absolute configurations of compounds 8 and 9 were assigned by experimental and theoretically calculated electronic
Paul E Harrington et al.
Current medicinal chemistry, 14(28), 3027-3034 (2008-01-29)
The calcium sensing receptor (CaR) is a G protein-coupled receptor (GPCR) that plays a fundamental role in serum calcium homeostasis. The CaR is expressed on the chief cells of the parathyroid gland and is responsible for controlling the secretion of
Chromatograms
application for HPLCHPLC Analysis of α-Methylbenzylamine Enantiomers (DANSYL Derivatives) on Astec® CYCLOBOND I 2000 DMP
application for HPLC我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门