推荐产品
化驗
99%
形狀
liquid
折射率
n20/D 1.526 (lit.)
bp
185 °C/756 mmHg (lit.)
密度
0.94 g/mL at 25 °C (lit.)
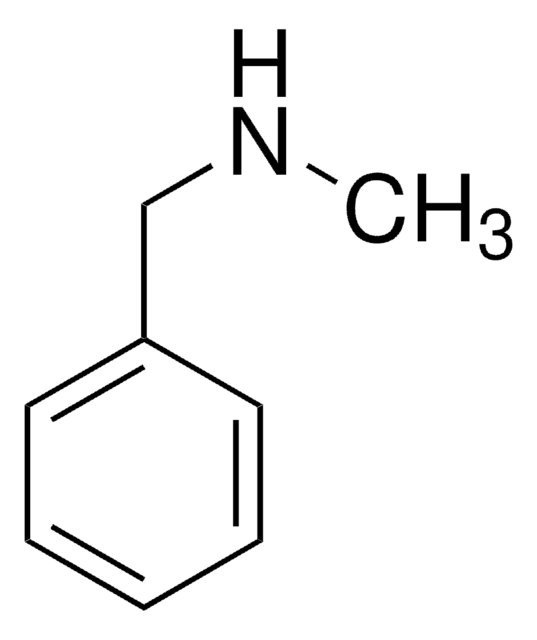
SMILES 字串
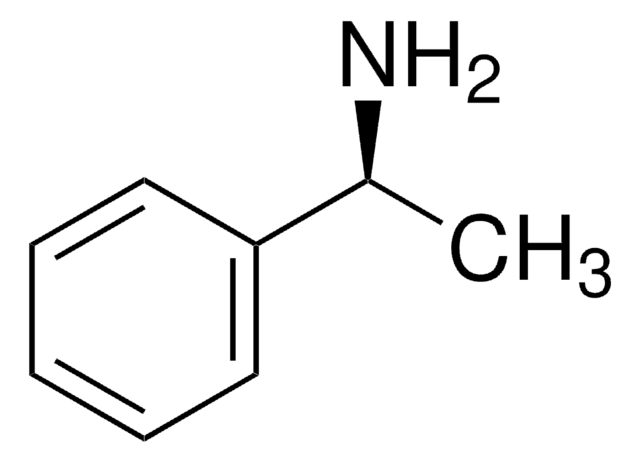
CC(N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3
InChI 密鑰
RQEUFEKYXDPUSK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
α-甲基苄胺是一种氮源,被广泛用作研究伯胺化学酶动力学拆分的一种代表性底物。
應用
α-甲基苄胺可通过与外消旋双酚衍生物进行反应,用作合成二氢-5H-二苯并[c,e]氮杂环丙烷盐的反应物。
它可用于合成 o-氟苄胺。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
158.0 °F - closed cup
閃點(°C)
70 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Boyeong Kang et al.
Scientific reports, 7(1), 4786-4786 (2017-07-08)
The energy flow during natural photosynthesis is controlled by maintaining the spatial arrangement of pigments, employing helices as scaffolds. In this study, we have developed porphyrin-peptoid (pigment-helix) conjugates (PPCs) that can modulate the donor-acceptor energy transfer efficiency with exceptional precision
Chemoenzymatic dynamic kinetic resolution of primary amines.
Paetzold, Jens and Backvall, Jan E
Journal of the American Chemical Society, 127(50), 17620-17621 (2005)
Nora Weber et al.
Microbial cell factories, 16(1), 3-3 (2017-01-05)
Whole-cell biocatalysis based on metabolically active baker's yeast with engineered transamination activity can be used to generate molecules carrying a chiral amine moiety. A prerequisite is though to express efficient ω-transaminases and to reach sufficient intracellular precursor levels. Herein, the
Palladium-Catalyzed ortho-Selective C-H Fluorination of Oxalyl Amide-Protected Benzylamines.
Chen, Changpeng et al.
The Journal of Organic Chemistry, 80(2), 942-949 (2014)
Jan Dines Knudsen et al.
Microbial cell factories, 15, 37-37 (2016-02-18)
Saccharomyces cerevisiae can be engineered to perform a multitude of different chemical reactions that are not programmed in its original genetic code. It has a large potential to function as whole-cell biocatalyst for one-pot multistep synthesis of various organic molecules
Chromatograms
HPLC Analysis of α-Methylbenzylamine Enantiomers (DANSYL Derivatives) on Astec® CYCLOBOND I 2000 DMP
application for HPLCapplication for HPLCGlobal Trade Item Number
| 货号 | GTIN |
|---|---|
| M31104-10L | |
| M31104-100ML | 4061834046363 |
| M31104-500ML | 4061836830991 |
| M31104-25ML | 4061834046370 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持