推荐产品
品質等級
化驗
≥98%
mp
104-106 °C (lit.)
溶解度
acetone: 25 mg/mL, clear, colorless
官能基
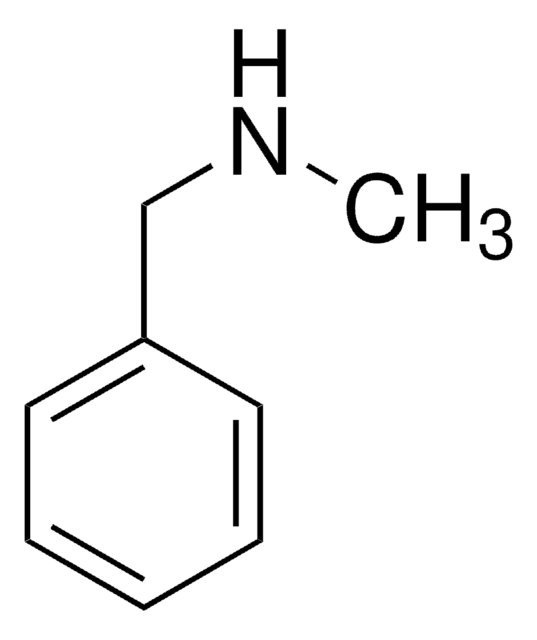
amide
phenyl
SMILES 字串
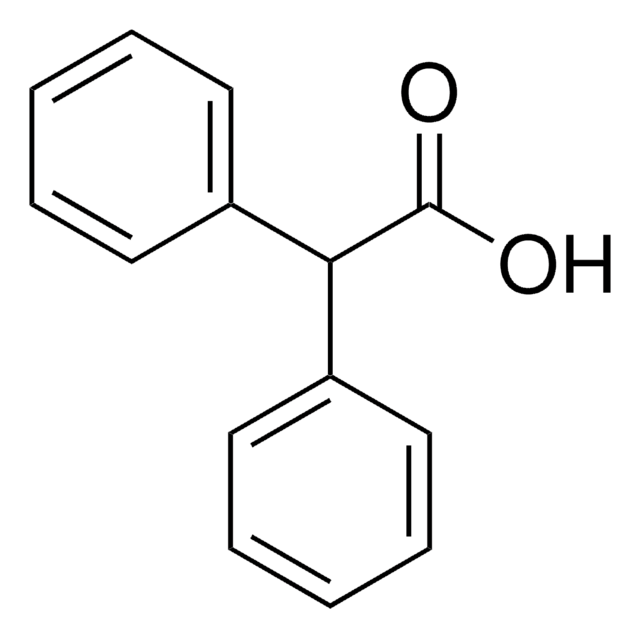
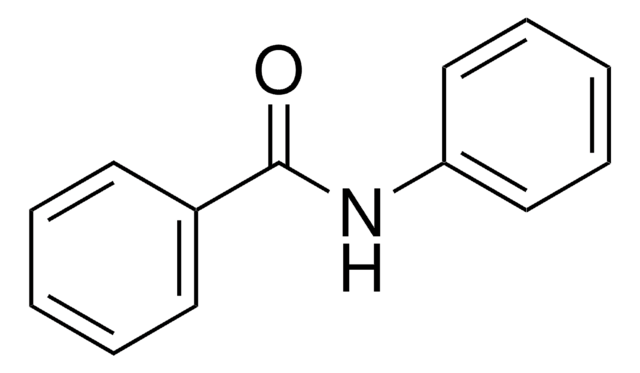
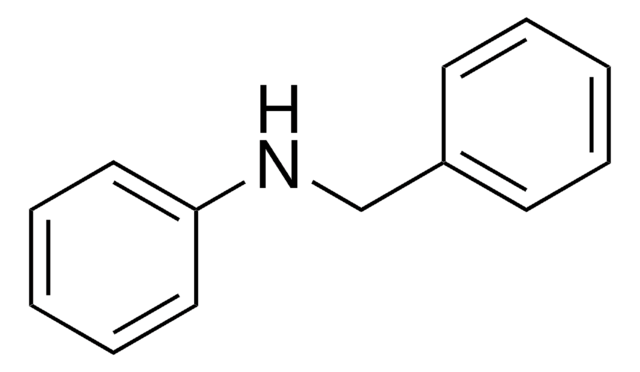
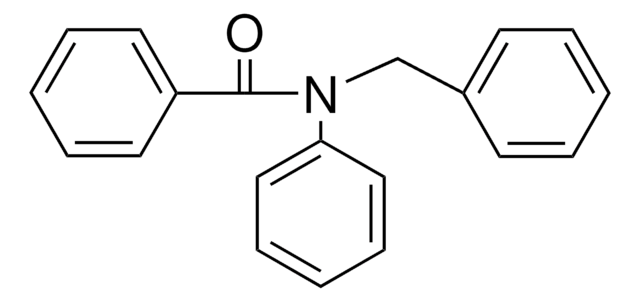
O=C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H13NO/c16-14(13-9-5-2-6-10-13)15-11-12-7-3-1-4-8-12/h1-10H,11H2,(H,15,16)
InChI 密鑰
LKQUCICFTHBFAL-UHFFFAOYSA-N
一般說明
N-苄基苯甲酰胺抑制酪氨酸酶的活性。
應用
α 取代苄胺的便捷前体,用于滴定丁基锂和其他锂碱的指示剂。相关参考文献,请参见 Aldrichimica Acta 。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
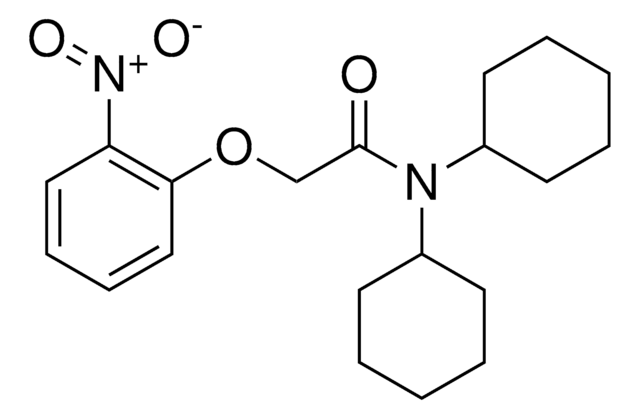
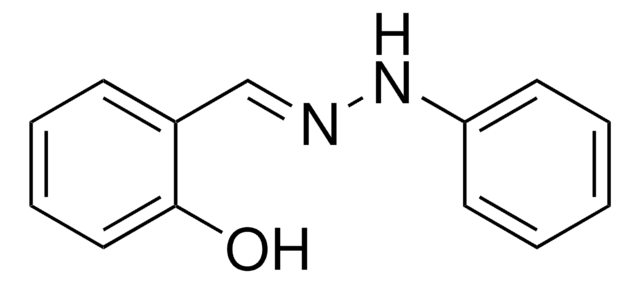
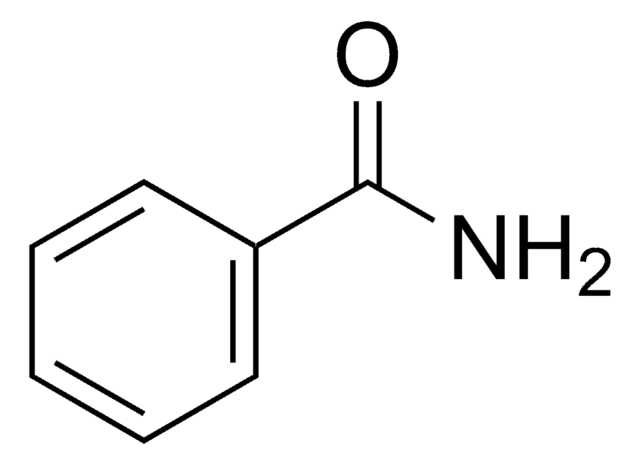
其他客户在看
Aldrichimica Acta, 11, 20-20 (1978)
Yae Eun Chong et al.
Biomedical chromatography : BMC, 33(11), e4653-e4653 (2019-07-20)
Ondansetron, a widely used antiemetic agent, is a P-glycoprotein (P-gp) substrate and therefore expression of P-gp at the blood-brain barrier limits its distribution to the central nervous system (CNS), which was observed to be reversed by coadministration with P-gp inhibitors.
Zsanett Dorkó et al.
Talanta, 162, 167-173 (2016-11-14)
A simple and efficient method is presented for assessing molecularly imprinted polymers (MIP) and other sorbents from the point of view of practical applications. The adsorption isotherms of the compounds, which need to be separated or detected in an application
Sung Jin Cho et al.
Bioorganic & medicinal chemistry letters, 16(10), 2682-2684 (2006-03-04)
A series of potent inhibitors of tyrosinase and their structure-activity relationships are described. N-Benzylbenzamide derivatives (1-21) with hydroxyl(s) were synthesized and tested for their tyrosinase inhibitory activity. With this series, compound 15 provided a potent tyrosinase inhibition: it effectively inhibited
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门