所有图片(1)
About This Item
经验公式(希尔记法):
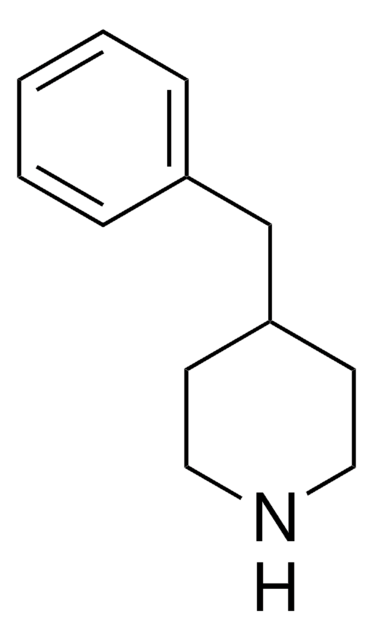
C12H18N2
CAS号:
分子量:
190.28
Beilstein:
146038
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.543 (lit.)
密度
0.933 g/mL at 25 °C (lit.)
SMILES 字串
NC1CCN(CC1)Cc2ccccc2
InChI
1S/C12H18N2/c13-12-6-8-14(9-7-12)10-11-4-2-1-3-5-11/h1-5,12H,6-10,13H2
InChI 密鑰
YUBDLZGUSSWQSS-UHFFFAOYSA-N
基因資訊
rat ... Grin2a(24409)
正在寻找类似产品? 访问 产品对比指南
應用
4-Amino-1-benzylpiperidine was used in the preparation of:
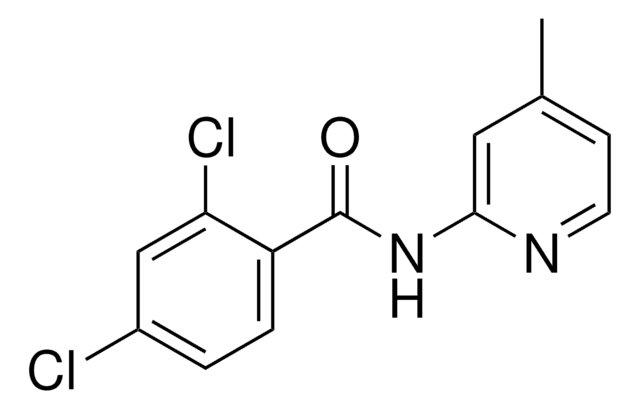
- butyl 4-amino-1-piperidineacetate, key intermediate required for the synthesis of butyl 4-[(4-amino-5-chloro-2-methoxybenzoyl)amino]-1-piperidineacetate
- 5-alkylimino-1,2,4-thiadiazolidine-3-ones, such as 4-ethyl-5-[imino-[1-(phenylmethyl)-4-piperidinyl]]-2-methyl-1,2,4-thiadiazolidin-3-one and 4-benzyl-5-[imino-[1-(phenylmethyl)-4-piperidinyl]]-2-isopropyl-1,2,4-thiadiazolidin-3-one
- glycyrrhetinic acid derivatives
Reactant for synthesis of:
Highly selective inhibitors of p38a mitogen-activated protein kinase
Antiplasmodial compounds
Dual activity cholinesterase and Aβ-aggregation inhibitors
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonists
Photostable near-infrared cyanine dyes
99mTc-labeled piperidine analogues for targeting sigma receptors
Highly selective inhibitors of p38a mitogen-activated protein kinase
Antiplasmodial compounds
Dual activity cholinesterase and Aβ-aggregation inhibitors
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonists
Photostable near-infrared cyanine dyes
99mTc-labeled piperidine analogues for targeting sigma receptors
訊號詞
Warning
危險聲明
危險分類
Skin Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
J Sakaguchi et al.
Chemical & pharmaceutical bulletin, 49(6), 788-790 (2001-06-20)
A new and facile route for the synthesis of the novel gastrointestinal prokinetic butyl 4-[(4-amino-5-chloro-2-methoxybenzoyl)amino]-1-piperidineacetate (1b), which exhibited potent gastro- and colon-prokinetic activities by oral administration without significant side effects, was established. The key intermediate, butyl 4-amino-1-piperidineacetate (16), was prepared
A Martinez et al.
European journal of medicinal chemistry, 35(10), 913-922 (2000-12-21)
A new family of 1,2,4-thiadiazolidinone derivatives containing the N-benzylpiperidine fragment has been synthesised. The acetylcholinesterase (AChE) inhibitory activity of all compounds was measured using Ellman's method and some of them turned out to be as potent as tacrine. Furthermore, compound
Federica Prati et al.
ChemMedChem, 11(12), 1284-1295 (2016-02-18)
We discovered a small series of hit compounds that show multitargeting activities against key targets in Alzheimer's disease (AD). The compounds were designed by combining the structural features of the anti-AD drug donepezil with clioquinol, which is able to chelate
Soo-Jong Um et al.
Bioorganic & medicinal chemistry, 11(24), 5345-5352 (2003-12-04)
To synthesize glycyrrhetinic acid (GA) derivatives (3, 4, 5, 10, 13, 14, 15, and 16), we first removed the ketonic group in the C-11 position, and the carboxylic function at the C-30 position was kept intact, reduced to an alcohol
Josef Dib et al.
Journal of mass spectrometry : JMS, 50(2), 407-417 (2015-03-25)
AdipoR agonists are small, orally active molecules capable of mimicking the protein adiponectin, which represents an adipokine with antidiabetic and antiatherogenic effects. Two adiponectin receptors were reported in the literature referred to as adipoR1 and adipoR2. Activation of these receptors
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门