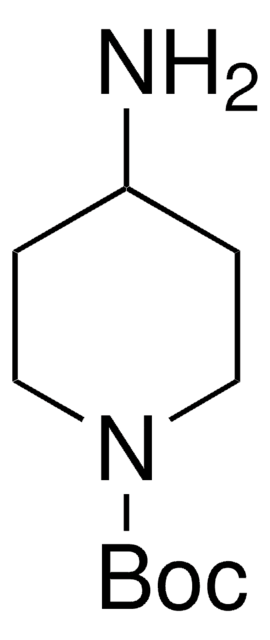
所有图片(1)
About This Item
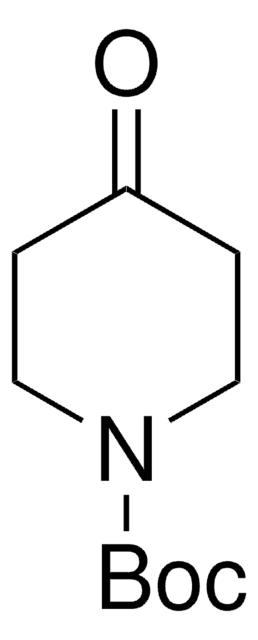
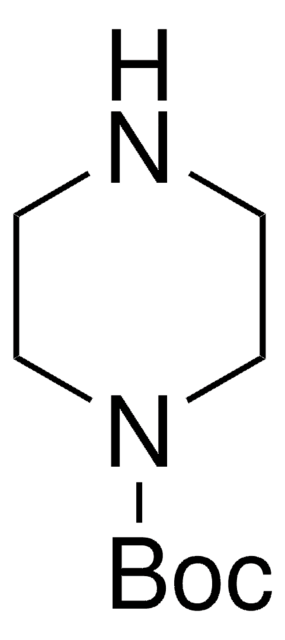
经验公式(希尔记法):
C10H20N2O2
CAS号:
分子量:
200.28
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
96%
mp
162-166 °C (lit.)
官能基
amine
SMILES 字串
CC(C)(C)OC(=O)NC1CCNCC1
InChI
1S/C10H20N2O2/c1-10(2,3)14-9(13)12-8-4-6-11-7-5-8/h8,11H,4-7H2,1-3H3,(H,12,13)
InChI 密鑰
CKXZPVPIDOJLLM-UHFFFAOYSA-N
一般說明
4-(N-Boc-amino)piperidone is a piperidone derivative.
應用
制药结构单元。
用于合成具有强效抗艾滋病病毒 (HIV-1) 活性的哌啶-4-甲酰胺趋化因子受体 5 (CCR5) 拮抗剂。
4-(N-Boc-amino)piperidone may be used as a functionalization reagent for introduction of a primary and a tertiary amino group to poly(2-isopropyl-2-oxazoline.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Shinichi Imamura et al.
Journal of medicinal chemistry, 49(9), 2784-2793 (2006-04-28)
We incorporated various polar groups into previously described piperidine-4-carboxamide CCR5 antagonists to improve their metabolic stability in human hepatic microsomes. Introducing a carbamoyl group into the phenyl ring of the 4-benzylpiperidine moiety afforded the less lipophilic compound 5f, which possessed
Poly (2-isopropyl-2-oxazoline)-poly (L-glutamate) block copolymers through ammonium-mediated NCA polymerization.
Meyer M and Schlaad H.
Macromolecules, 39(11), 3967-3970 (2006)
Apos Dermatakis et al.
Bioorganic & medicinal chemistry, 11(8), 1873-1881 (2003-03-28)
A series of oxindole CDK2 inhibitors was synthesized. These novel analogues have a saturated monosubstituted cyclic moiety at their C-4 position that mimics the ribofuranoside of ATP. This substitution afforded agents with increased potency relative to the parent indolinone and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门