推荐产品
化驗
97%
形狀
powder
mp
103-108 °C (lit.)
SMILES 字串
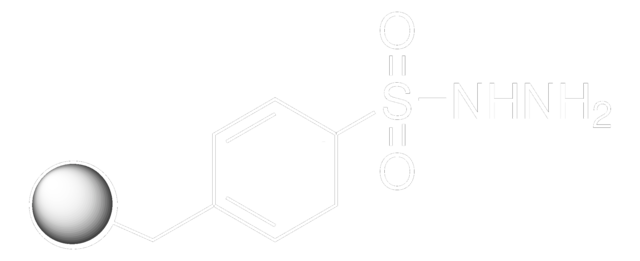
Cc1ccc(cc1)S(=O)(=O)NN
InChI
1S/C7H10N2O2S/c1-6-2-4-7(5-3-6)12(10,11)9-8/h2-5,9H,8H2,1H3
InChI 密鑰
ICGLPKIVTVWCFT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
对甲苯磺酰肼可用作制备甲苯磺酰腙的试剂。其可用于制备二吡唑并[1,5-a:4′,3′-c]吡啶 和1,2,3-硒二唑衍生物。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Aquatic Chronic 2 - Self-react. D
安全危害
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Journal of the Chemical Society. Perkin Transactions 1, 945-945 (1993)
Journal of the American Chemical Society, 114, 966-966 (1992)
Mousa Al-Smadi et al.
Molecules (Basel, Switzerland), 13(11), 2740-2749 (2008-11-07)
The commercially available aromatic polyketones 1a-d were utilized for the synthesis of the multi-arm1,2,3-selenadiazole derivatives 3a-d. The preparation starts with the reaction between compounds 1a-d and p-toluenesulfonyl hydrazide to give the corresponding tosylhydrazones 2a-d. Subsequent reaction with selenium dioxide leads
Dipyrazolo[1,5-a:4',3'-c]pyridines - a new heterocyclic system accessed via multicomponent reaction.
Wolfgang Holzer et al.
Beilstein journal of organic chemistry, 8, 2223-2229 (2013-02-01)
The synthesis of dipyrazolo[1,5-a:4',3'-c]pyridines is described. Easily obtainable 5-alkynylpyrazole-4-carbaldehydes, p-toluenesulfonyl hydrazide, and an aldehyde or ketone containing an α-hydrogen atom were reacted in a silver triflate catalyzed multicomponent reaction affording new tricyclic compounds with a dipyrazolo[1,5-a:4',3'-c]pyridine core. Detailed NMR spectroscopic
Journal of the American Chemical Society, 115, 2473-2473 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门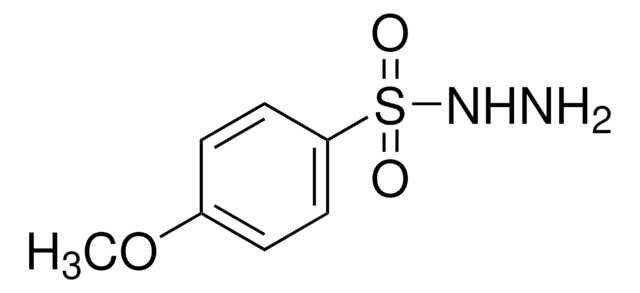
![1H-1,2,3-三唑并[4,5-b]吡啶 98%](/deepweb/assets/sigmaaldrich/product/structures/344/744/1e7fa2cf-1258-48e0-909f-92509981f43d/640/1e7fa2cf-1258-48e0-909f-92509981f43d.png)