Ion Exchange Chromatography Troubleshooting
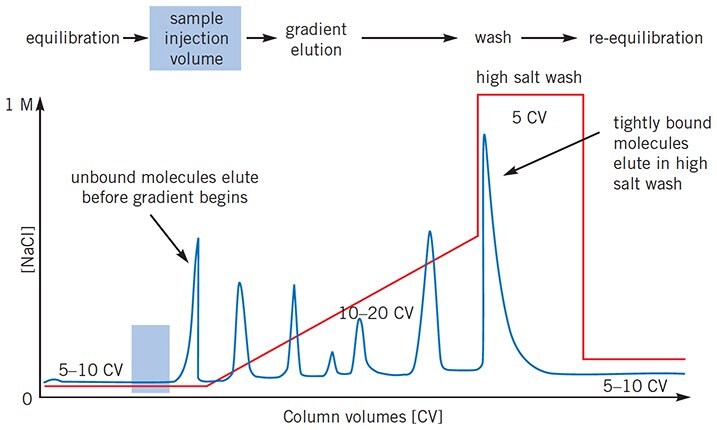
Figure 1.The ideal IEX separation: target proteins well resolved by gradient elution
If only certain peaks are of interest in this well-resolved separation, it may be advantageous to transfer to a step elution in order to save time and buffer. The rest of this section focuses on practical problems that may lead to a non-ideal IEX separation.

Figure 2.Sample elutes before salt gradient begins
Ensure that buffers are in the correct containers. Reduce ionic strength of sample by desalting, page 156, or dilution with start buffer. For an anion exchanger, increase buffer pH, for a cation exchanger, decrease buffer pH. If proteins still do not bind at any pH, it is possible that the column has been contaminated by detergent.
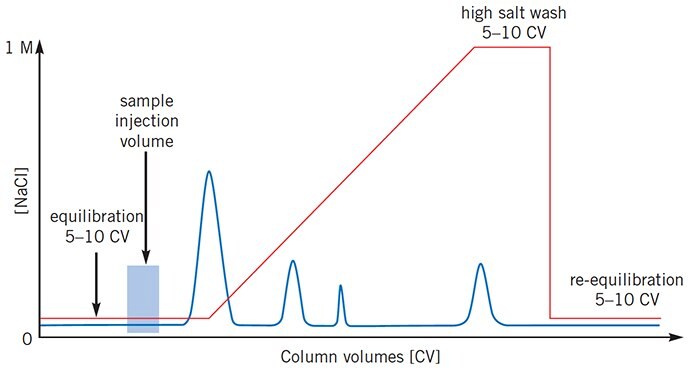
Figure 3.Sample still eluting when gradient begins
After sample application the UV trace must return to baseline before elution begins, otherwise proteins that do not bind to the column interfere with the separation. Increase the volume of start buffer (equilibration step) before starting the gradient elution.
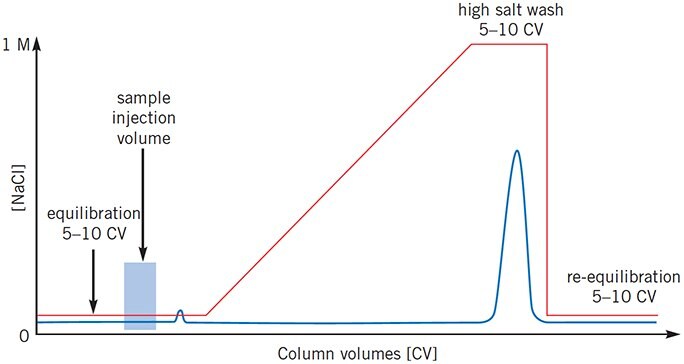
Figure 4.Sample elutes during high salt wash
Proteins are binding too strongly. Ensure that buffers are in the correct containers. If using an anion exchanger, decrease buffer pH, if using a cation exchanger, increase buffer pH.
Protein(s) of interest eluting late in gradient
Proteins are binding too strongly. Increase ionic strength of gradient. It is preferable to alter pH if a very high salt concentration is required for elution. For an anion exchanger, decrease buffer pH and for a cation exchanger, increase buffer pH. Refer also to Table 6.
Protein(s) of interest eluting too early in gradient:
Proteins are not binding strongly. Check ionic strength of gradient. Alter pH, for an anion exchanger, increase buffer pH and for a cation exchanger, decrease buffer pH. Refer also to Table 6.
Proteins(s) of interest not sufficiently resolved
Refer to the contents of this chapter to review key parameters for improving resolution. Refer also to Table 6.
*Polar organic solvents such as methanol, ethanol, isopropanol and acetonitrile can be used at concentrations from 0–20%, but remember that some proteins may irreversibly lose their biological activity in the presence of organic solvents. Check sample and buffer solubility, buffer pH and chemical stability of the medium before running a column.
Note that back pressure may increase when working with organic solutions.
To continue reading please sign in or create an account.
Don't Have An Account?