Performing a Separation with Cytiva Products Based on Protein A
Protein A is derived from a strain of Staphylococcus aureus and contains five regions that bind to the Fc region of IgG. As an affinity ligand, protein A is coupled to Sepharose so that these regions are free to bind IgG. One molecule of protein A can bind at least two molecules of IgG.
Both protein A and a recombinant protein A are available from Cytiva. These molecules share similar specificities for the Fc region of IgG, but the recombinant protein A has been engineered to include a C-terminal cysteine that enables a single-point coupling to Sepharose. Single point coupling often results in an enhanced binding capacity.
The binding strength of protein A for IgG depends on the source species of the immunoglobulin as well as the subclass of IgG (Table 2). The dynamic binding capacity depends on the binding strength and also on several other factors, such as flow rate during sample application.
Although IgG is the major reactive human immunoglobulin, some other types have also been demonstrated to bind to protein A. Interaction takes place with human colostral IgA as well as human myeloma IgA2 but not IgA1. Some human monoclonal IgMs and some IgMs from normal and macroglobulinaemic sera can bind to protein A.
Leakage of ligands from an affinity medium is always a possibility, especially if harsh elution conditions are used. The multi-point attachment of protein A to Sepharose results in very low leakage levels over a wide range of elution conditions.
Purifcation Options |
|---|
* Protein A Sepharose 4 Fast Flow and rProtein A Sepharose Fast Flow have a higher binding capacity, a more rigid matrix and provide more convenient alternatives to Protein A Sepharose CL-4B, which must be rehydrated before column packing.
** See Appendix 4 to convert linear flow (cm/h) to volumetric flow rate. Maximum operating flow is calculated from measurement in a packed column with a bed height of 10 cm and i.d. of 5 cm.
Purification Examples
Figure 17 shows the purification of mouse IgG2b from ascites fluid on HiTrap® rProtein A FF 1 mL column using a syringe. The eluted pool contained 1 mg IgG2b and the silver stained SDS-PAGE gel confirmed a purity level of over 95%.
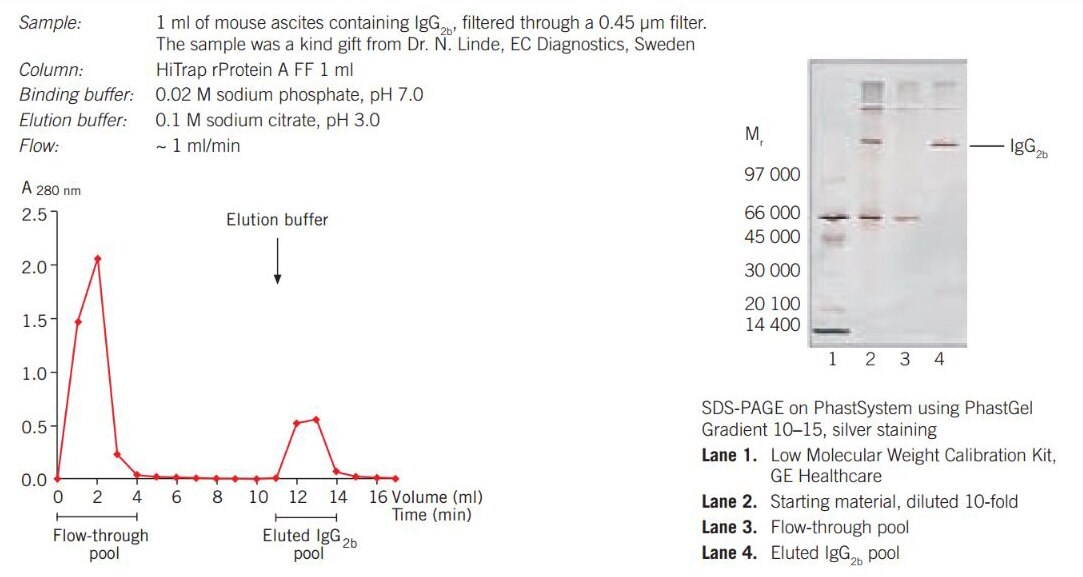
Figure 17.Purifcation of mouse IgG2b from ascites on HiTrap® rProtein A FF 1 mL column using a syringe.
Figure 18 shows a larger scale purification of monoclonal mouse IgG2a from a clarified hybridoma cell culture on rProtein A Sepharose Fast Flow. Sample loading was over 9 mg IgG/mL of medium, with a 95% recovery of highly purified antibody.
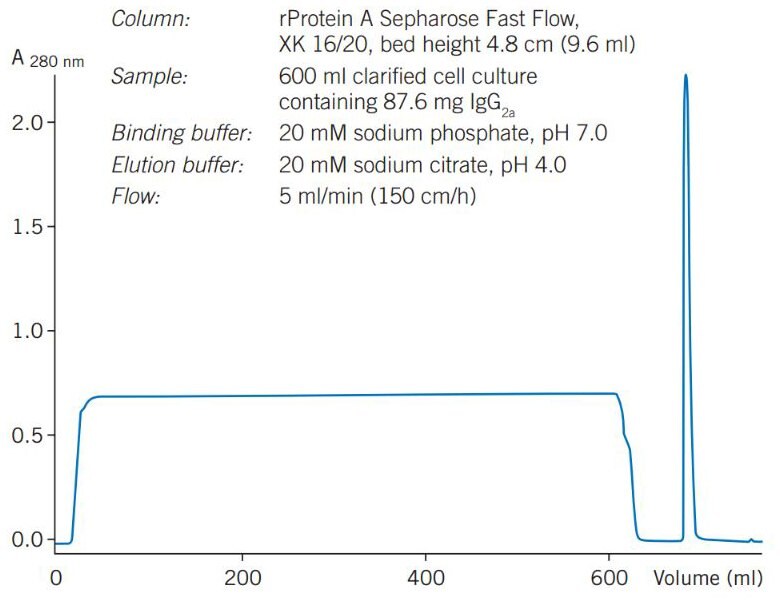
Figure 18a.Purification of a monoclonal IgG2a from clarified cell culture on rProtein A Sepharose 4 Fast Flow.
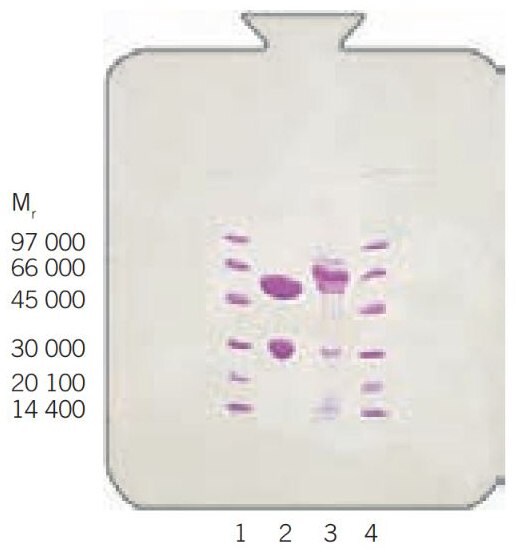
Figure 18b.SDS-PAGE of starting material (lane 2) and eluate (lane 3). The samples were concentrated 10 times and reduced. Lane 1 and 4 are LMW markers. PhastSystem, PhastGel Gradient 10–15.
Performing a Separation
Column: HiTrap® Protein A HP, 1 mL or 5 mL, or HiTrap® rProtein A FF, 1 mL or 5 mL
Recommended flow rates: 1 mL/min (1 mL columns) or 5 mL/min (5 mL columns)
Binding buffer: 0.02 M sodium phosphate, pH 7.0
Elution buffer: 0.1 M citric acid, pH 3–6
Neutralization buffer: 1 M Tris-HCl, pH 9.0
Centrifuge samples (10 000 g for 10 minutes) to remove cells and debris. Filter through a 0.45 µm filter. If needed, adjust sample conditions to the pH and ionic strength of the binding buffer either by buffer exchange on a desalting column (page 133, Buffer exchange and desalting for affinity chromatography) or by dilution and pH adjustment.
A HiTrap® column can be used with a syringe, a peristaltic pump or connected to a liquid chromatography system, such as ÄKTAprime plus.
- Equilibrate the column with 5 column volumes of binding buffer.
- Apply sample.
- Wash with 5–10 column volumes of the binding buffer to remove impurities and unbound material. Continue until no protein is detected in the eluent (determined by UV absorbance at 280 nm).
- Elute with 5 column volumes of elution buffer.*
- Immediately re-equilibrate with 5–10 column volumes of binding buffer.
* Since elution conditions are quite harsh, collect fractions into neutralization buffer (60 µL – 200 µL 1 M Tris-HCl, pH 9.0 per mL fraction), so that the final pH of the fractions will be approximately neutral.
Table 3 gives examples of some typical binding and elution conditions that have been used with Protein A Sepharose.
Table 3 |
|---|
Binding strengths are tested with free protein A. They can be used as a guide to predict the binding behaviour to a protein A affinity medium. However, when coupled to an affinity matrix the interaction may be altered. For example, rat IgG1 does not bind to protein A, but does bind to Protein A Sepharose.
IgGs from most species and subclasses bind protein A near to physiological pH and ionic strength. Avoid excessive washing if the interaction between the protein of interest and the ligand is weak, since this may decrease the yield.
With some antibodies, such as mouse IgG1, it might be necessary to add sodium chloride up to 3 M in the binding buffer to achieve efficient binding when using protein A, for example 1.5 M glycine, 3 M NaCl, pH 8.9.
Alternative elution buffers include: 1 M acetic acid, pH 3.0 or 0.1 M glycine-HCl, pH 3.0 or 3 M potassium isothiocyanate.
Potassium isothiocyanate can severely affect structure and immunological activity.
Use a mild elution method when labile antibodies are isolated. Reverse the flow of the wash buffer and elute with 0.1 M glycyltyrosine in 2 M NaCl, pH 7.0 at room temperature, applied in pulses. (Note: glycyltyrosine absorbs strongly at wavelengths used for detecting proteins). The specific elution is so mild that the purified IgG is unlikely to be denatured.
To increase capacity, connect several HiTrap® Protein A HP or HiTrap® rProtein A FF columns (1 mL or 5 mL) in series or pack a larger column with Protein A Sepharose 4 Fast Flow or rProtein A Sepharose 4 Fast Flow (Appendix 3, Column packing and preparation for affinity chromatography).
Desalt and/or transfer purified IgG fractions into a suitable buffer using a desalting column (see page 133, Buffer exchange and desalting for affinity chromatography).
Reuse of Protein A Sepharose and rProtein A Sepharose media depends on the nature of the sample and should only be considered when processing identical samples to avoid cross-contamination.
Media Characteristics |
|---|
* Long term refers to the pH interval over which the medium is stable over a long period of time without adverse effects on its subsequent chromatographic performance. Short term refers to the pH interval for regeneration, cleaning-i lace and sanitization procedures.
** Protein A Sepharose 4 Fast Flow and rProtein A Sepharose 4 Fast Flow have a higher binding capacity, a more rigid matrix and provide more convenient alternatives to Protein A Sepharose CL-4B which must be rehydrated before column packing.
Chemical Stability
These media and columns tolerate high concentrations of urea, guanidine HCl and chaotropic agents.
Storage
Wash media and columns with 20% ethanol (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
To continue reading please sign in or create an account.
Don't Have An Account?