1A00800
USP
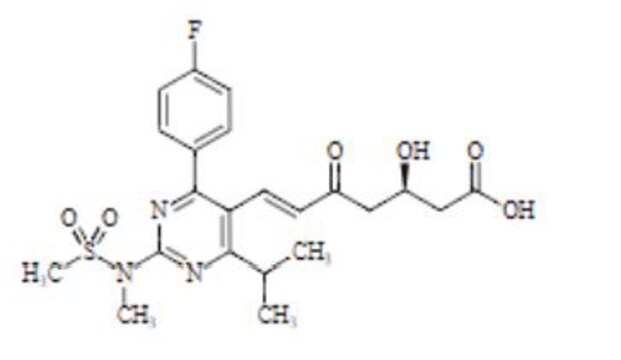
ATORVASTATIN EPOXY TETRAHYDROFURAN ANALOG
Pharmaceutical Analytical Impurity (PAI)
Synonym(s):
4-(4-Fluorophenyl)-2,4-dihydroxy-2-(1-methylethyl)-N,5-diphenyl-3,6-dioxabicyclo[3.1.0]hexane-1-carboxamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical analytical impurity (PAI)
agency
USP
API family
atorvastatin
manufacturer/tradename
USP
application(s)
pharmaceutical
format
neat
storage temp.
2-8°C
General description
ATORVASTATIN EPOXY TETRAHYDROFURAN ANALOG is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Atorvastatin Calcium
Therapeutic Area: Antihyperlipidemics
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Atorvastatin Calcium
Therapeutic Area: Antihyperlipidemics
For more information about this PAI, visit here.
Application
ATORVASTATIN EPOXY TETRAHYDROFURAN ANALOG (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
Features and Benefits
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Analysis Note
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Other Notes
Sales restrictions may apply.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service