G1006621
GC Analysis of Terpenes in Cannabis on Equity-1 after Headspace SPME using 50/30 μm DVB/CAR/PDMS Fiber
suitable for GC/MS, suitable for solid phase microextraction (SPME)
About This Item
Recommended Products
technique(s)
GC/MS: suitable
solid phase microextraction (SPME): suitable
test parameters
sample/matrix: 0.5 g dried cannabis in 10 mL headspace vial
SPME fiber: Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS), 50/30 μm (57298-U)
extraction: 20 min, headspace, 40 °C
desorption process: 3 min, 270 °C
sample preparation: 30 min equilibration at 40 °C prior to extraction
sample preparation: fiber postbake after extraction, 3 min at 270 °C
column: Equity-1, 60 m x 0.25 mm I.D., 0.25 μm (28047-U)
oven: 60 °C (2 min), 5 °C/min to 275 °C (5 min)
inj. temp.: 270 °C
detector: MSD
MSD interface: 300 °C
carrier gas: helium, 1 mL/min constant flow
liner: 0.75 mm ID, SPME
application(s)
food and beverages
vitamins, nutraceuticals, and natural products
Analysis Note
Legal Information
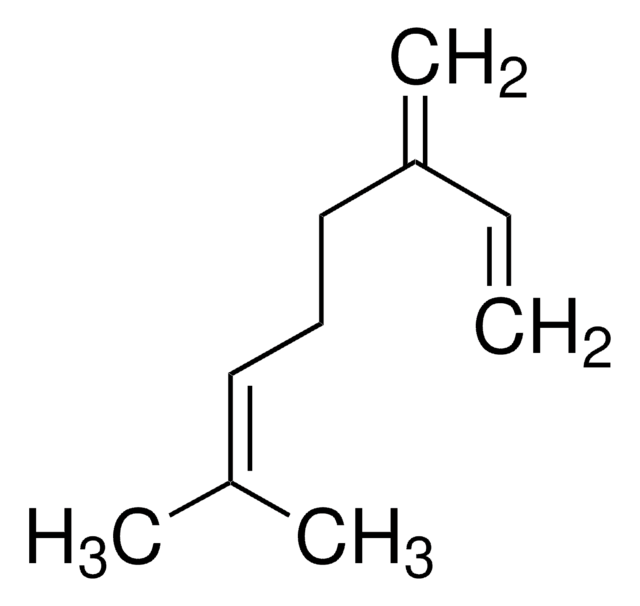
Analyte
Analytical column
SPME fiber
accessory
standard
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service