T7063
Tryptase from human lung
buffered aqueous solution, ≥5 units/mg protein
Synonym(s):
Tryptase enzyme
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
buffered aqueous solution
Quality Level
specific activity
≥5 units/mg protein
mol wt
~135 kDa
shipped in
dry ice
storage temp.
−20°C
Gene Information
human ... TPSAB1(7177) , TPSB2(64499) , TPSD1(23430) , TPSG1(25823)
General description
Tryptase is a glycoprotein released from mast cells during anaphylaxis, which performs a number of functions including catalyzing the activation of complement C3, converting prostromelysin to stromelysin (MMP-3), and cleaving fibrinogen resulting in a loss of clotting potential. Human tryptase is a major secretory protease of human lung mast cells.
Application
Tryptase has been used in a study that purified and characterized recombinant rat mast cell protease 7 expressed in Pichia pastoris. Tryptase has also been used in a study to investigate drug allergies in mast cell disease.
Biochem/physiol Actions
Tryptase is a member of the serine protease S1 family. It is the predominant neutral protease of the mast cell granules. Within the mast cell granule it exists as a heparin-stabilized active tetramer. Stabilization is a result of the high negative charge density of the glycosaminoglycan. This stabilization activity is observed with heparins with a MW greater than 6 kDa as well as other glycosaminoglycans such as dextran sulfate or chondroitin sulfates. Removal of heparin results in dissociation of the tetramer and inactivation of the enzyme. High concentrations of NaCl will result in the dissociation of heparin.
Tryptase is released from the mast cell as a result of the degranulation response during anaphylaxis. In addition, several tryptase genes and alleles (α, β, γ & δ) have been identified in various tissues and circulating in blood. Pro-β-tryptase is thought to be the constituative circulating form in blood.
The biological function of tryptase is unknown. However it has been reported to catalyze the activation of complement C3, convert prostromelysin to stromelysin (MMP-3), and cleave fibrinogen resulting in a loss of clottting potential. Tryptase also degrades fibronectin, calcitonin gene-related peptide, vasoactive intestinal peptide,
and kininogen.
Tryptase is released from the mast cell as a result of the degranulation response during anaphylaxis. In addition, several tryptase genes and alleles (α, β, γ & δ) have been identified in various tissues and circulating in blood. Pro-β-tryptase is thought to be the constituative circulating form in blood.
The biological function of tryptase is unknown. However it has been reported to catalyze the activation of complement C3, convert prostromelysin to stromelysin (MMP-3), and cleave fibrinogen resulting in a loss of clottting potential. Tryptase also degrades fibronectin, calcitonin gene-related peptide, vasoactive intestinal peptide,
and kininogen.
Quality
Highly purified
Physical properties
Molecular Weight: ~135 kDa (Human)(Non-covalently linked tetramer with two sets of dissimilar subunits possibly resulting from heterogeneity in N-linked glycosylation and existence of a & b isoforms sequences in human lung). 31-33 kDa (Monomer MW)
Unit Definition
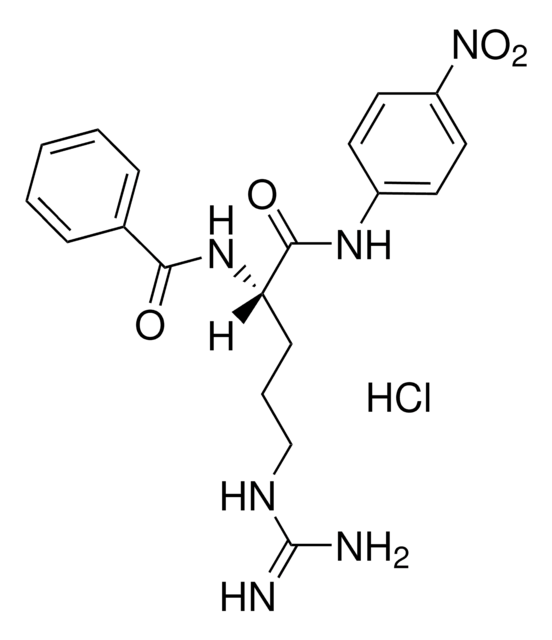
One unit will hydrolyze 1.0 μmole of N-benzyl-DL-Arg-pNA per minute at pH 8 at 25 °C.
Physical form
Supplied as a liquid in 50mM Sodium Acetate, 1M Sodium Chloride, pH 5.0 with 0.05mM Heparin and 0.01% Sodium Azide
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ali Zare-Mirzaie et al.
Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association, 18(5), 322-326 (2012-09-26)
Chronic diarrhea is defined as a decrease in fecal consistency lasting for four or more weeks. Prevalence of this complication in the general population is 5%. Mast cells that play an important role in the regulation of gastrointestinal visceral sensitivity
Manuela Torres Andion Vidal et al.
Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 34(1), 309-316 (2012-10-23)
The aim of this study was to investigate the density of mast cells and microvessels in minor salivary gland tumors. Forty-one cases of minor salivary gland tumors (pleomorphic adenoma, n = 10; adenoid cystic carcinoma, n = 11; mucoepidermoid carcinoma, n = 10; and polymorphous low-grade
T J Smith et al.
The Journal of biological chemistry, 259(17), 11046-11051 (1984-09-10)
Human lung tryptase, a mast cell-derived trypsin-like serine protease, has been isolated from whole human lung tissue obtained at autopsy. Increased yields from this purification process have allowed extensive characterization of the enzyme. One of the critical steps in the
Liang Chen et al.
Journal of cellular biochemistry, 120(6), 9799-9809 (2018-12-16)
Macrophages polarization plays essential but different roles in most diseases such as atherosclerosis, adipose tissue inflammation, and insulin resistance. Our previous study revealed that protease-activated receptor 2 (PAR2), a G-protein coupled receptor influenced macrophage function, but little is known regarding
Michele Ammendola et al.
Updates in surgery, 65(1), 53-57 (2012-11-03)
Literature data indicate that mast cells (MCs) are involved in angiogenesis through the release of several pro-angiogenetic factors among which tryptase, a serine protease stored in MC granules, is one of the most active. However, no data are available concerning
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service