SAE0172
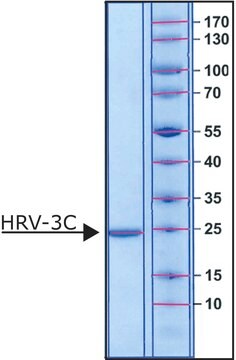
Mpro, 3CL Protease from coronavirus SARS-COV-2
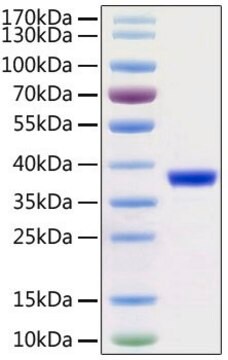
recombinant protein, lyophilized product
Synonym(s):
2019-nCoV, 3C Like proteinase, 3C-like main protease, 3CL Mpro, 3CL protease, 3CL proteinase, 3CLpro, COVID-19, COVID-2019, SARS-CoV-2, coronavirus
About This Item
Recommended Products
General description
- Mpro (main protease)
- PLpro (Papain-like protease)
SARS-CoV Mpro, 3CL exists as a homodimer and each protomer has an active site. The proteolytic cleavage of 1ab polyprotein by Mpro occurs at 11 sites: 7 sites within the 1a polyprotein, and 11 sites within the 1ab polyprotein. This results in maturation of 16 viral non-structural proteins.Mpro protease forms a functional homodimer. Both the N-terminus and the C- terminus of Mpro have been shown to be critical for dimer formation and for enzyme function.
The Mpro protease, 3CLpro is an ideal target for antiviral drug design due to its high conservation between different coronavirus strains and absence of functional analogs in the human proteome. Mpro protease from SARS-CoV1 and SARS-CoV2 are functionally identical.
Features and Benefits
Physical form
The product is supplied lyophilized from 20 mM HEPES, 2.5% Trehalose, and 0.05% Tween 20
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service