S7129
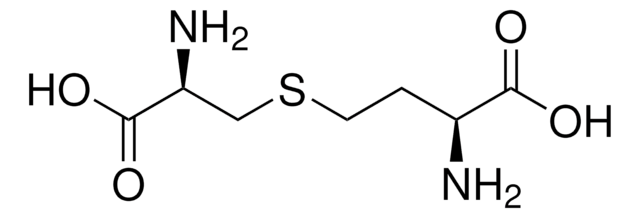
O-Succinyl-L-homoserine
≥98.0% (TLC), suitable for ligand binding assays
Synonym(s):
L-Homoserine 4-(hydrogen butanedioate)
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H13NO6
CAS Number:
Molecular Weight:
219.19
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
O-Succinyl-L-homoserine,
assay
≥98.0% (TLC)
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
N[C@@H](CCOC(=O)CCC(O)=O)C(O)=O
InChI
1S/C8H13NO6/c9-5(8(13)14)3-4-15-7(12)2-1-6(10)11/h5H,1-4,9H2,(H,10,11)(H,13,14)/t5-/m0/s1
InChI key
GNISQJGXJIDKDJ-YFKPBYRVSA-N
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P Vermeij et al.
Journal of bacteriology, 181(18), 5833-5837 (1999-09-11)
Cysteine and methionine biosynthesis was studied in Pseudomonas putida S-313 and Pseudomonas aeruginosa PAO1. Both these organisms used direct sulfhydrylation of O-succinylhomoserine for the synthesis of methionine but also contained substantial levels of O-acetylserine sulfhydrylase (cysteine synthase) activity. The enzymes
T Clausen et al.
The EMBO journal, 17(23), 6827-6838 (1998-12-08)
The transsulfuration enzyme cystathionine gamma-synthase (CGS) catalyses the pyridoxal 5'-phosphate (PLP)-dependent gamma-replacement of O-succinyl-L-homoserine and L-cysteine, yielding L-cystathionine. The crystal structure of the Escherichia coli enzyme has been solved by molecular replacement with the known structure of cystathionine beta-lyase (CBL)
Susan M Aitken et al.
Biochemistry, 42(38), 11297-11306 (2003-09-25)
Cystathionine gamma-synthase (CGS) is a pyridoxal phosphate-dependent enzyme that catalyzes a gamma-replacement reaction, in which the succinyl group of an O-succinyl-L-homoserine (L-OSHS) is displaced by the thiol of L-cysteine to form L-cystathionine, in the first step of the bacterial transsulfuration
M Foglino et al.
Microbiology (Reading, England), 141 ( Pt 2), 431-439 (1995-02-01)
The relationship between genes and enzymes in the methionine biosynthetic pathway has been studied in Pseudomonas aeruginosa. The first step is catalysed by an O-succinylhomoserine synthase, the product of the metA gene mapped at 20 min on the chromosome. The
Susan M Aitken et al.
Archives of biochemistry and biophysics, 433(1), 166-175 (2004-12-08)
The ability of enzymes to catalyze specific reactions, while excluding others, is central to cellular metabolism. Control of reaction specificity is of particular importance for enzymes that employ catalytically versatile cofactors, of which pyridoxal 5'-phosphate is a prime example. Cystathionine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service