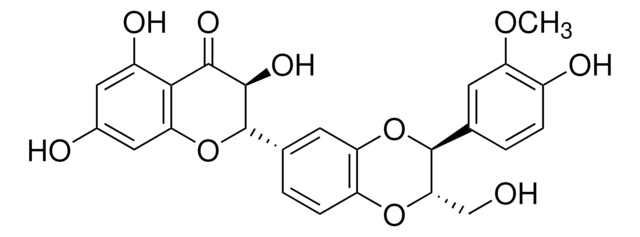
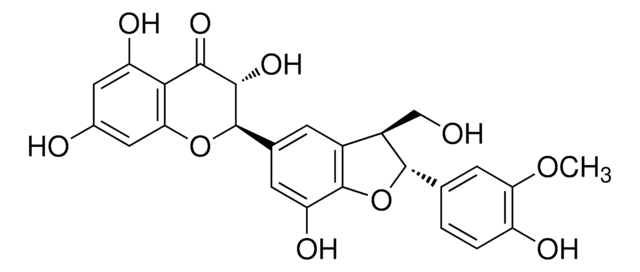
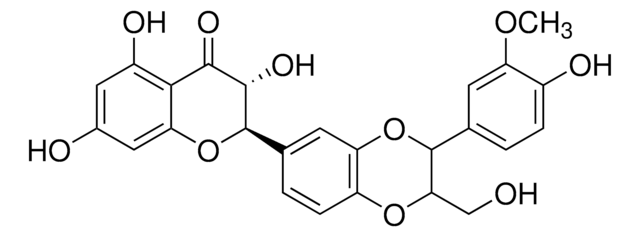
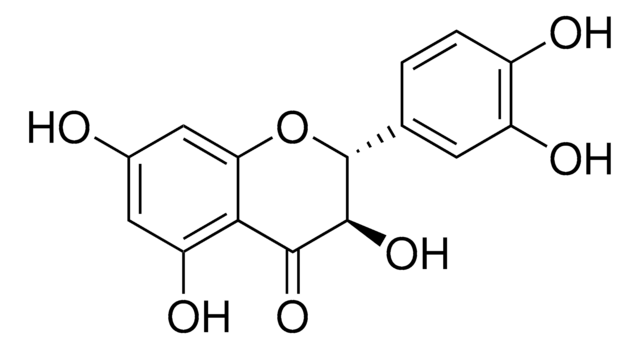
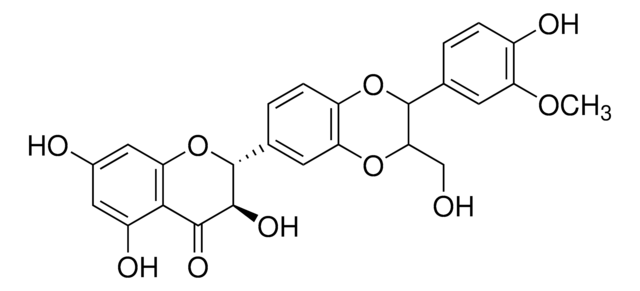
S0292
Silymarin
flavonolignans
Synonym(s):
flavolignans
About This Item
Recommended Products
biological source
plant (Silybum marianum)
Quality Level
form
powder
technique(s)
toxicology assay: suitable
application(s)
microbiology
storage temp.
−20°C
InChI
1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3
InChI key
SEBFKMXJBCUCAI-UHFFFAOYSA-N
General description
Application
- its in vitro antiviral, antibacterial, antifungal activities and cytotoxicity
- its effect of silymarin on bladder contractions in cyclophosphamide (CYP)-induced cystitis rat model
- its effect on liver toxication induced by Fumonisin B1 in mice
Biochem/physiol Actions
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service