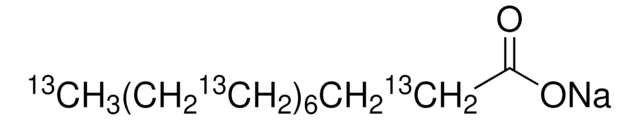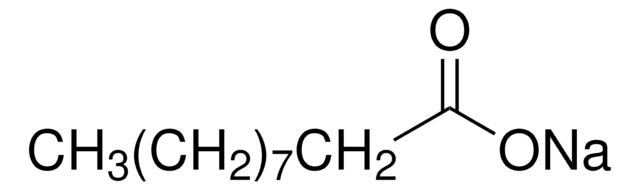P9767
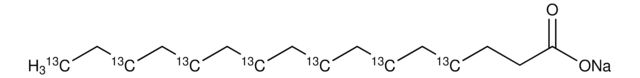
Sodium palmitate
≥98.5%
Synonym(s):
Hexadecanoic acid sodium salt, Palmitic acid sodium salt
About This Item
Recommended Products
biological source
plant (palm)
Quality Level
assay
≥98.5%
form
powder
mp
283-290 °C (lit.)
functional group
ester
lipid type
saturated FAs
shipped in
ambient
storage temp.
2-8°C
SMILES string
[Na+].CCCCCCCCCCCCCCCC([O-])=O
InChI
1S/C16H32O2.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18;/h2-15H2,1H3,(H,17,18);/q;+1/p-1
InChI key
GGXKEBACDBNFAF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- to induce inflammation and thrombosis pathway in murine macrophage cell line RAW 264.7 cell line by activating reactive oxygen species (ROS) production, Janus-kinase (JNK) signalling and release of histone H3 by western blotting and cell viability by MTT assay
- to induce lipogenesis in AML12 cells and primary hepatocytes to analyse the effect of irisin on PA induced lipogenesis and related signal pathways by western blot analysis and quantitative PCR analysis
- as a component in free fatty acid mixture to induce cellular steatosis in HepG2 cell lines and determination of lipid accumulation by Oil-Red-O staining
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service