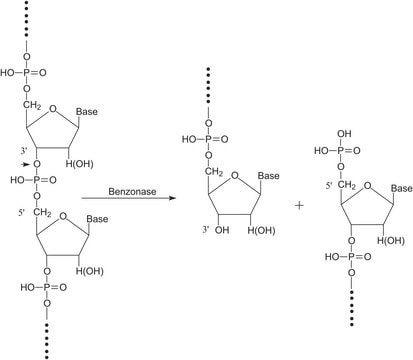
P8849
Protease Inhibitor Cocktail
DMSO solution, for the inhibition of serine, cysteine, aspartic, aminopeptidases and thermolysin-like activities, for use in purification of Histidine-tagged proteins, DMSO solution
Synonym(s):
Protease inhibitor solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
form:
DMSO solution
solubility:
water: soluble
storage temp.:
−20°C
Recommended Products
Product Name
Protease Inhibitor Cocktail, for use in purification of Histidine-tagged proteins, DMSO solution
biological source
synthetic
Quality Level
form
DMSO solution
solubility
water: soluble
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
DMSO solution designed for use in the purification of histidine-tagged proteins.
The cocktail contains a mixture of inhibitors that specifically target serine, cysteine, aspartic, and thermolysin-like proteases, and aminopeptidases.
The cocktail contains a mixture of inhibitors that specifically target serine, cysteine, aspartic, and thermolysin-like proteases, and aminopeptidases.
Specificity
Inhibits serine, cysteine, aspartic, and thermolysin-like proteases, and aminopeptidases.
Application
The cocktail has been optimized and tested for isolation of histidine-tagged proteins, with chelating agents omitted for compatibility with IMAC applications.
It is recommended for inhibition of protease activity in 100 mL of cell lysate from 20 g of Escherichia coli or 10 g of baculovirus-infected Spodoptera frugiperda pupal ovary cells.
It is recommended for inhibition of protease activity in 100 mL of cell lysate from 20 g of Escherichia coli or 10 g of baculovirus-infected Spodoptera frugiperda pupal ovary cells.
Features and Benefits
Specifically formulated for histidine-tagged protein purification.
Targets multiple types of proteases, ensuring comprehensive inhibition of protease activity.
Compatibility with IMAC applications due to omission of chelating agents.
Supplied in convenient packaging options.
Targets multiple types of proteases, ensuring comprehensive inhibition of protease activity.
Compatibility with IMAC applications due to omission of chelating agents.
Supplied in convenient packaging options.
Components
AEBSF
Bestatin
E-64
Pepstatin A
Phosphoramidon
Bestatin
E-64
Pepstatin A
Phosphoramidon
Quantity
One mL is recommended for the inhibition of proteases extracted from 20 g of Escherichia coli or 10 g of baculovirus-infected Spodoptera frugiperda pupal ovary cells in a total volume of 100 ml.
This protease inhibitor cocktail has been optimized and tested for histidine-tagged proteins
This protease inhibitor cocktail has been optimized and tested for histidine-tagged proteins
Preparation Note
This product is supplied as a clear solution in DMSO. One mL of solution is recommended for inhibition of protease activity in 100 mL of cell lysate from 20 g of E. coli cells or 10 g of baculovirus-infected cells.
Related product
Product No.
Description
Pricing
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
185.0 °F - closed cup
flash_point_c
85 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Rivier et al.
The EMBO journal, 20(7), 1765-1773 (2001-04-04)
In Chlamydomonas reinhardtii, the psaA mRNA is assembled by a process involving trans-splicing of separate transcripts, encoded at three separate loci of the chloroplast genome. At least 14 nuclear loci and one chloroplast gene, tscA, are needed for this process.
Intracellular Nogo-A facilitates initiation of neurite formation in mouse midbrain neurons in vitro.
Z Kurowska et al.
Neuroscience, 256, 456-466 (2013-10-26)
Nogo-A is a transmembrane protein originally discovered in myelin, produced by postnatal CNS oligodendrocytes. Nogo-A induces growth cone collapse and inhibition of axonal growth in the injured adult CNS. In the intact CNS, Nogo-A functions as a negative regulator of
Shuji Kaneko et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 22(1), 82-92 (2002-01-05)
The physical interaction between the presynaptic vesicle release complex and the large cytoplasmic region linking domains II and III of N-type (Ca(v)2.2) calcium channel alpha(1)B subunits is considered to be of fundamental importance for efficient neurotransmission. By PCR analysis of
Shahar Rotem-Bamberger et al.
PloS one, 8(3), e58470-e58470 (2013-03-09)
ASPP2 is a key protein in regulating apoptosis both in p53-dependent and-independent pathways. The C-terminal part of ASPP2 contains four ankyrin repeats and an SH3 domain (Ank-SH3) that mediate the interactions of ASPP2 with apoptosis related proteins such as p53
E Boudreau et al.
The EMBO journal, 19(13), 3366-3376 (2000-07-06)
The psbD mRNA, which encodes the D2 reaction center polypeptide of photosystem II, is one of the most abundant chloroplast mRNAs. We have used genomic complementation to isolate the nuclear Nac2 gene, which is required for the stable accumulation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service