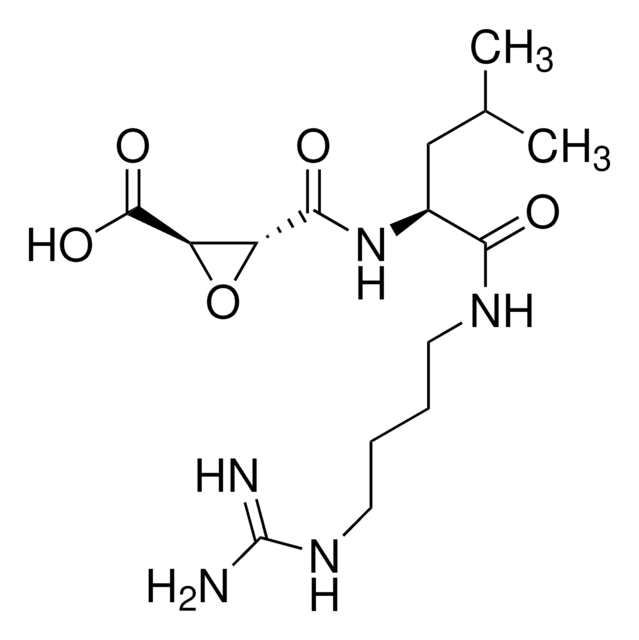
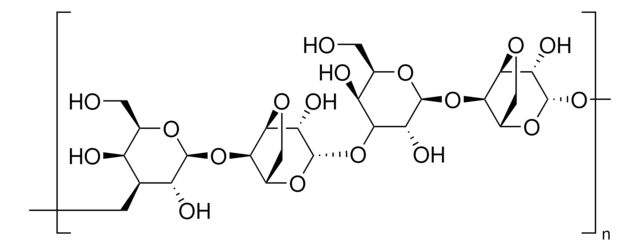
P2032
Pepstatin A−Agarose
saline suspension
Synonym(s):
Pepstatin A resin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
microbial (fermentation)
plant
Quality Level
form
saline suspension
technique(s)
affinity chromatography: suitable
matrix
cross-linked 4% beaded agarose
matrix activation
cyanogen bromide
matrix attachment
carboxyl
matrix spacer
9 atoms
capacity
20-40 mg/mL binding capacity (pepsin)
suitability
suitable for chromatography
storage temp.
2-8°C
Application
Pepstatin A-agarose is used in protein chromatography, affinity chromatography and specialty resins. Pepstatin A-agarose has been used to characterize three chitosanase isozymes isolated from a commercial crude porcine pepsin preparation.
Physical form
Suspension in 0.5 M NaCl containing preservative
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P Geldhof et al.
International journal for parasitology, 33(2), 129-136 (2003-03-14)
A pepstatin A-agarose column was used in an attempt to purify a previously described antibody-degrading aspartyl proteinase from excretory-secretory material from the L4 and the adult stages of the bovine abomasal nematode Ostertagia ostertagi. However, no aspartyl proteinase activity was
N Hiraiwa et al.
European journal of biochemistry, 246(1), 133-141 (1997-05-15)
To understand the mechanism of the maturation of various proteins in protein-storage vacuoles, we purified a 48-kDa aspartic endopeptidase composed of 32-kDa and 16-kDa subunits from castor bean. Immunocytochemical and cell fractionation analyses of the endosperm of maturing castor bean
C J Morrison et al.
Journal of general microbiology, 139 Pt 6, 1177-1186 (1993-06-01)
Aspartyl proteinase (AP) is an extracellular enzyme of Candida albicans implicated as a pathogenic factor. Previous reports on the purification and characterization of AP suggested that a single DEAE-Sephadex chromatographic step was sufficient for the removal of extraneous proteins and
Liliana Rojo et al.
Marine biotechnology (New York, N.Y.), 12(6), 696-707 (2010-02-20)
Acid digestive proteinases were studied in the gastric fluids of two species of clawed lobster (Homarus americanus and Homarus gammarus). An active protein was identified in both species as aspartic proteinase by specific inhibition with pepstatin A. It was confirmed
O Carnevali et al.
Biology of reproduction, 60(1), 140-146 (1998-12-22)
Oocyte growth within the follicle is preponderantly due to the accumulation of hepatically derived yolk protein (vitellogenin, VTG) by receptor-mediated endocytosis; once in the oocyte, VTG is partially processed and stored in yolk globules. In some pelagic egg-laying marine teleosts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service