I4883
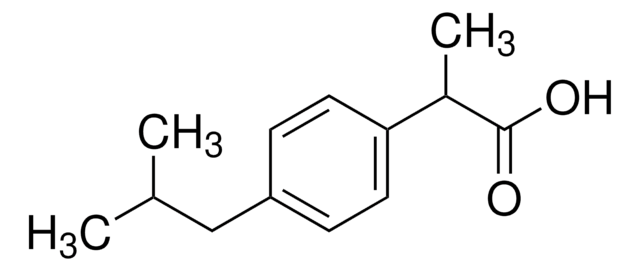
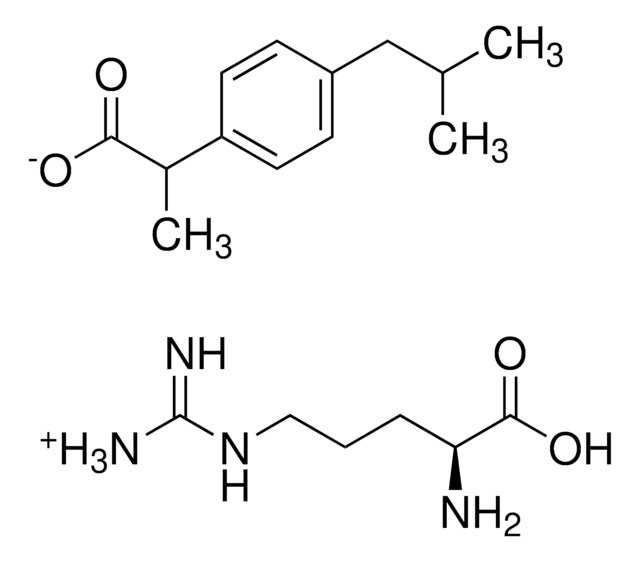
Ibuprofen
≥98% (GC), powder, COX inhibitor
Synonym(s):
2-(4-Isobutylphenyl)propanoic acid, Brufen, Motrin, Rebugen, α-Methyl-4-(isobutyl)phenylacetic acid, (±)-2-(4-Isobutylphenyl)propanoic acid
About This Item
Recommended Products
product name
Ibuprofen, ≥98% (GC)
biological source
synthetic (organic)
assay
≥98% (GC)
form
powder
mp
77-78 °C
solubility
ethanol: 50 mg/mL, clear, colorless to faintly yellow
SMILES string
CC(C)Cc1ccc(cc1)C(C)C(O)=O
InChI
1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
InChI key
HEFNNWSXXWATRW-UHFFFAOYSA-N
Gene Information
human ... ALB(213) , ALOX5(240) , CYP1A2(1544) , CYP2C9(1559) , IL8RA(3577) , PTGS1(5742) , PTGS2(5743)
mouse ... Alox5(11689)
rat ... Alox5(25290) , Ptgs1(24693)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its vascular and pulmonary effects on neonatal lung development
- to study its effects on cell apoptosis, cell proliferation, and histology changes in human cholangiocarcinoma cell lines
- in the preparation of a terpene-based therapeutic deep eutectic system (THEDES) to investigate its physicochemical, antimicrobial, and anticancer properties
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover Bioactive Small Molecules for Lipid Signaling Research
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service