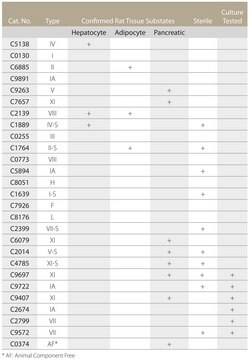
C2139
Collagenase from Clostridium histolyticum
suitable for release of rat epididymal adipocytes and hepatocytes (for methodology see Type II and Type IV), Type VIII, 0.5-5.0 FALGPA units/mg solid, ≥125 CDU/mg solid
Synonym(s):
Clostridiopeptidase A
About This Item
Recommended Products
biological source
Clostridium histolyticum
Quality Level
type
Type VIII
form
powder
specific activity
≥125 CDU/mg solid
0.5-5.0 FALGPA units/mg solid
mol wt
68-125 kDa
suitability
suitable for release of rat epididymal adipocytes and hepatocytes (for methodology see Type II and Type IV)
application(s)
diagnostic assay manufacturing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- for the preparation of arterial tissue for the study of advanced glycosylation end products (AGE)
- for use along with other proteases for the disaggregation of human tumor, mouse kidney, human brain, lung epithelium and many other tissues.
- in liver and kidney perfusion studies, digestion of pancreas, and isolation of nonparenchymal hepatocytes.
- for the preparation of viable hepatocytes from rat liver and for the isolation of fat cells from rat adipose tissue
Biochem/physiol Actions
Unit Definition
Analysis Note
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service