A9878
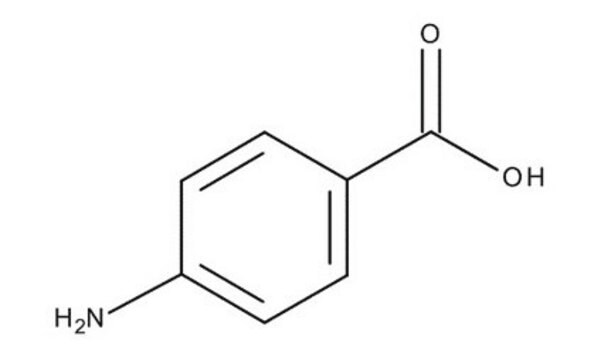
4-Aminobenzoic acid
≥99%, for peptide synthesis, ReagentPlus®
Synonym(s):
para-Aminobenzoic acid, PABA, Vitamin Bx, Vitamin H1
About This Item
Recommended Products
Product Name
4-Aminobenzoic acid, ReagentPlus®, ≥99%
biological source
synthetic (organic)
Quality Level
100
200
product line
ReagentPlus®
assay
≥99%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
187-189 °C (lit.)
solubility
ethanol: 50 mg/mL, clear, colorless to faintly yellow
density
1.374 g/mL at 25 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Nc1ccc(cc1)C(O)=O
InChI
1S/C7H7NO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H,9,10)
InChI key
ALYNCZNDIQEVRV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a building block in the synthesis of Polyamides
- As a substrate in folic acid production
- In the synthesis of Schiff base
- As an organic ligand in metal-organic framework (MOFs) synthesis
Legal Information
Not finding the right product?
Try our Product Selector Tool.
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 3
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
339.8 °F - closed cup
flash_point_c
171 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Importance and uses of folic acid in serum-free eukaryotic, including hybridoma and Chinese Hamster Ovary (CHO) cell, cultures. Folic acid and its reduced derivative(s), such as folinic acid, are an essential water soluble vitamins added to virtually all cell culture media.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service