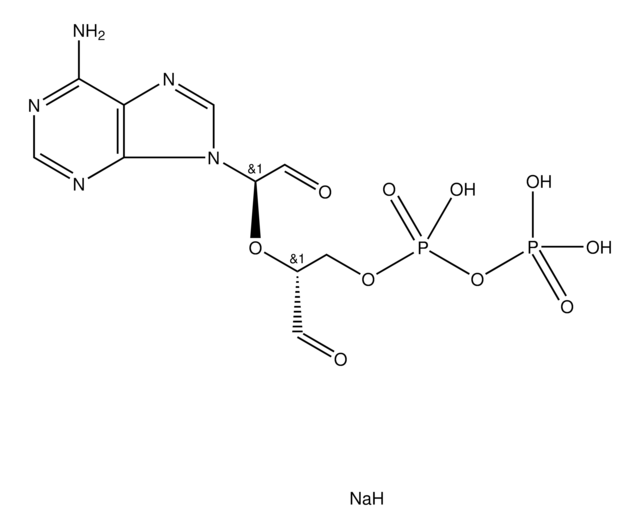
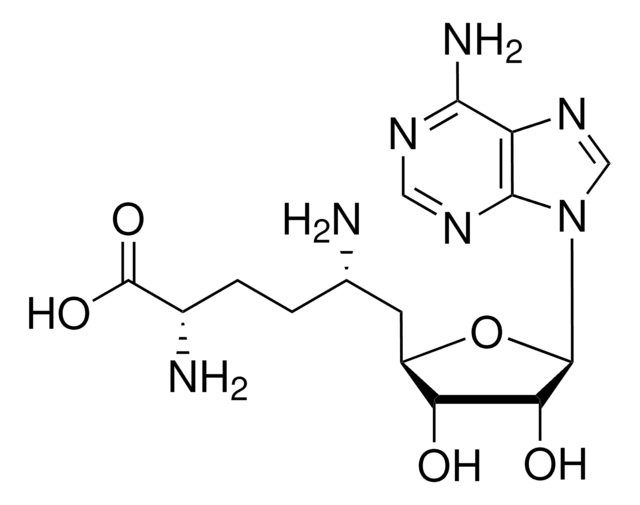
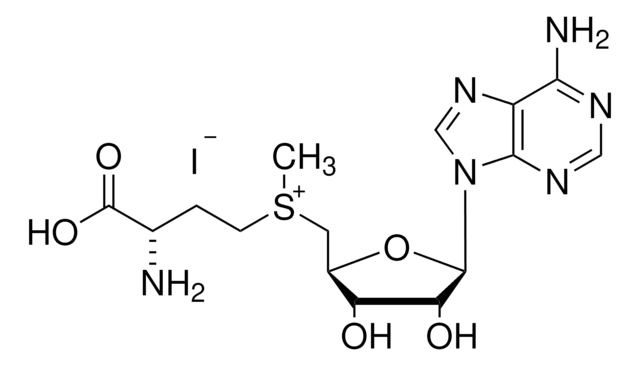
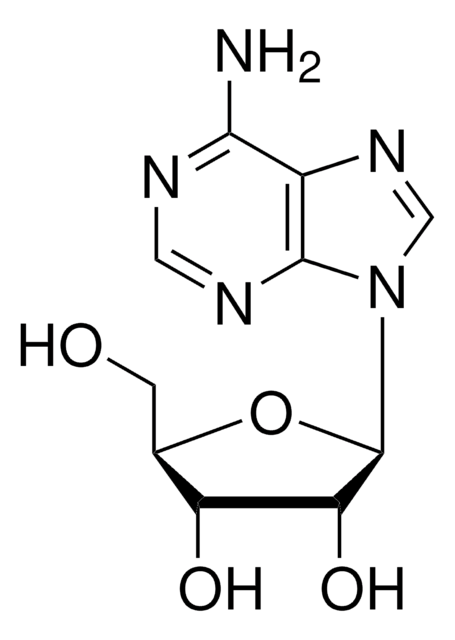
A7154
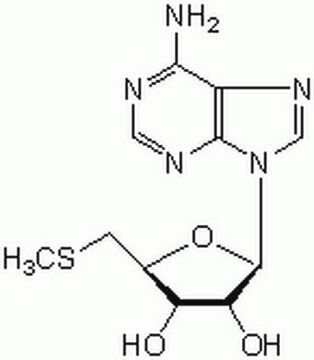
Adenosine, periodate oxidized
≥93%
Synonym(s):
ADOX, Adenosine-2′,3′-dialdehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H11N5O4
CAS Number:
Molecular Weight:
265.23
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
assay
≥93%
form
powder
solubility
0.2 M HCl: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
Nc1ncnc2n(cnc12)C(OC(CO)C=O)C=O
InChI
1S/C10H11N5O4/c11-9-8-10(13-4-12-9)15(5-14-8)7(3-18)19-6(1-16)2-17/h1,3-7,17H,2H2,(H2,11,12,13)
InChI key
ILMNSCQOSGKTNZ-UHFFFAOYSA-N
Application
Adenosine, periodate oxidized has been used:
- as a methylarginine transferase inhibitor in the human embryonic kidney (HEK)-293 T cells
- as a methylase inhibitor in H4 neuroglioma
- as a broad inhibitor of S-adenosylmethionine (AdoMet)-dependent methyltransferases in mouse embryo fibroblast NIH3T3 cells
Biochem/physiol Actions
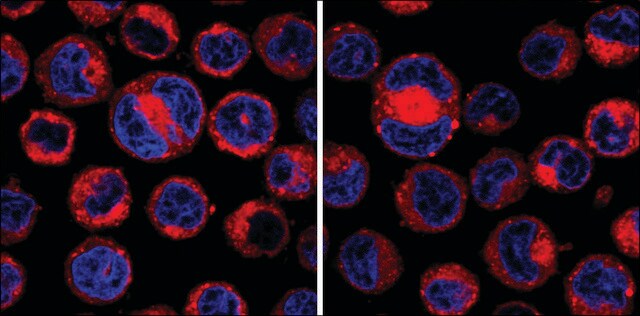
Adenosine, periodate oxidized (Adox) is a protein arginine methyltransferases (PRMTs) inhibitor. It also inhibits the enzyme S-adenosylhomocysteine hydrolase and induces apoptosis. Its inhibitory effect on histone methyltransferases prevents histone methylation. Adox also elicits intrinsic cytotoxic properties.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ryan Heit et al.
Journal of cell science, 122(Pt 16), 2957-2968 (2009-07-30)
Trimethylation of lysine 9 on histone H3 (H3K9me3) is known both to be necessary for proper chromosome segregation and to increase in late G2. We investigated the role of late G2 methylation, specifically in mitotic progression, by inhibiting methylation for
Christian Schwerk et al.
Oncogene, 24(47), 7002-7011 (2005-07-12)
We have analysed the importance of proper substrate methylation by S-adenosylmethionine-dependent methyltransferases for cell survival and cell cycle progression. We show that treatment of cells with the methyltransferase inhibitor adenosine dialdehyde (AdOx) causes cell cycle arrest and death in different
Olga Blifernez et al.
The Plant journal : for cell and molecular biology, 65(1), 119-130 (2010-12-24)
Methylation of protein arginines represents an important post-translational modification mechanism, which has so far primarily been characterized in mammalian cells. In this work, we successfully identified and characterized arginine methylation as a crucial type of post-translational modification in the activity
Björn Hultberg
Clinica chimica acta; international journal of clinical chemistry, 356(1-2), 117-124 (2005-05-05)
Many clinical and epidemiological studies show that mild hyperhomocysteinemia is associated with premature vascular disease. Information about the metabolism of homocysteine is therefore essential for an understanding of its role in atherogenesis, thereby enabling a modulation of that risk. In
Yinghong He et al.
Journal of translational medicine, 11, 14-14 (2013-01-16)
Pharmacologic reactivation of fetal hemoglobin expression is a promising strategy for treatment of sickle cell disease and β-thalassemia. The objective of this study was to investigate the effect of the methyl transferase inhibitor adenosine-2',3'-dialdehyde (Adox) on induction of human fetal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service