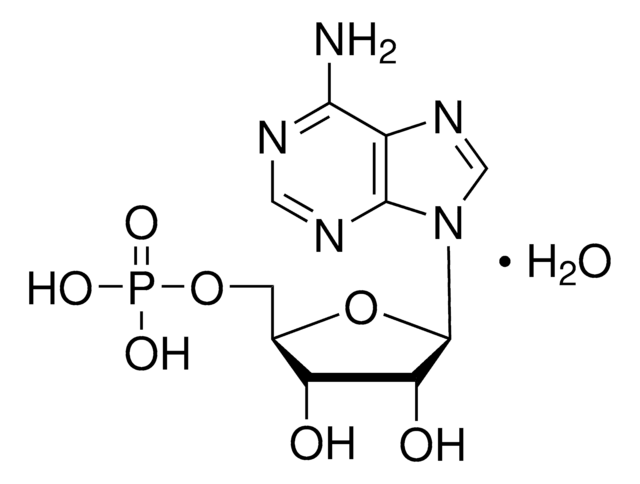
A1271
Adenosine 5′-monophosphate–Agarose
lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
41106500
eCl@ss:
32160414
PubChem Substance ID:
NACRES:
NA.56
Recommended Products
biological source
plant (Sea weed)
form
lyophilized powder
extent of labeling
1-5 μmol per mL
matrix
cross-linked 4% beaded agarose
matrix activation
cyanogen bromide
matrix attachment
C-8
matrix spacer
9 atoms
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Adenosine 5′-monophosphate Agarose (5′-AMP agarose) has been used in affinity chromatography to isolate β and gamma glutamate decarboxylase, which is important for controlling gamma-aminobutyric acid (GABA) synthesis in brain.
Physical form
Lyophilized powder stabilized with lactose
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
F James et al.
The Journal of biological chemistry, 270(38), 22344-22350 (1995-09-22)
The plant enzyme S-adenosylmethionine:methionine S-methyltransferase (EC 2.1.1.12, MMT) catalyzes the synthesis of S-methylmethionine. MMT was purified 620-fold to apparent homogeneity from leaves of Wollastonia biflora. The four-step purification included fractionation with polyethylene glycol, affinity chromatography on adenosine-agarose, anion exchange chromatography
C D Murphy et al.
Applied and environmental microbiology, 67(10), 4919-4921 (2001-09-26)
Streptomyces cattleya is unusual in that it produces fluoroacetate and 4-fluorothreonine as secondary metabolites. We now report the isolation of an NAD(+)-dependent fluoroacetaldehyde dehydrogenase from S. cattleya that mediates the oxidation of fluoroacetaldehyde to fluoroacetate. This is the first enzyme
S J Wu et al.
Journal of neurochemistry, 42(6), 1607-1612 (1984-06-01)
The interactions of two forms of porcine brain glutamate decarboxylase (beta-GAD and gamma-GAD) with the effector ATP were studied by affinity chromatography. A third form, alpha-GAD, was only slightly retarded by the affinity matrix and was eluted in the buffer
M Kato et al.
Plant physiology, 120(2), 579-586 (1999-06-11)
Caffeine synthase (CS), the S-adenosylmethionine-dependent N-methyltransferase involved in the last two steps of caffeine biosynthesis, was extracted from young tea (Camellia sinensis) leaves; the CS was purified 520-fold to apparent homogeneity and a final specific activity of 5.7 nkat mg-1
D L Martin et al.
Journal of neurochemistry, 55(2), 524-532 (1990-08-01)
A major regulatory feature of brain glutamate decarboxylase (GAD) is a cyclic reaction that controls the relative amounts of holoenzyme and apoenzyme [active and inactive GAD with and without bound pyridoxal 5'-phosphate (pyridoxal-P, the cofactor), respectively]. Previous studies have indicated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service