71010BC
Assurance® GDS Listeria monocytogenes Tq Kit
suitable for PCR testing in Food and Beverages applications
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
UNSPSC Code:
41106302
NACRES:
NA.85
Recommended Products
product name
GDS Listeria monocytogenes Tq, Molecular-based PCR assay suitable for detection of Listeria monocytogenes
agency
PCR, Immunomagnetic Separation (IMS)
AOAC® (PTM)
Health Canada
MicroVal
Quality Level
product line
Assurance GDS®
shelf life
6‑12 mo.
packaging
pkg of 100 tests
manufacturer/tradename
BioControl Systems 71010-100
technique(s)
PCR: suitable
application(s)
food and beverages
pathogen testing
compatibility
BioControl GDS
for use with GDS Rotor-Gene
storage temp.
2-8°C
suitability
Listeria monocytogenes
General description
Assurance® GDS, genetic detection system, for Listeria monocytogenes Tq is an automated nucleic acid amplification system for the detection of Listeria monocytogenes in food and environmental samples. The proprietary PickPen® IMS-based sample preparation procedure quickly and effectively captures Listeria monocytogenes, separating the pathogen from inhibitory background materials ensuring valid PCR reactions for all sample types, including challenging food and environmental samples. Assurance® GDS PCR technology provides two additional levels of specificity through the use of both highly specific primers and probes to ensure accurate detection of Listeria monocytogenes.
Application
GDS, genetic detection system, for Listeria monocytogenes Tq is an automated nucleic acid amplification system for the detection of Listeria monocytogenes in foods, ingredients, and environmental samples.
Features and Benefits
- Results in 24 hours for environmental samples and select foods.
- Single-step enrichment reduces handling time.
- Features innovative PickPen® immunomagnetic separation (IMS) for additional specificity and faster time to results.
- Simplified sample preparation does not require heating blocks or preparation of reagents.
- Proprietary Rotor-Gene® Q cycler is a rotory-based cycler that ensures uniform heating and cooling of all samples.
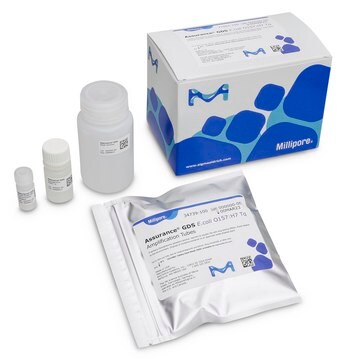
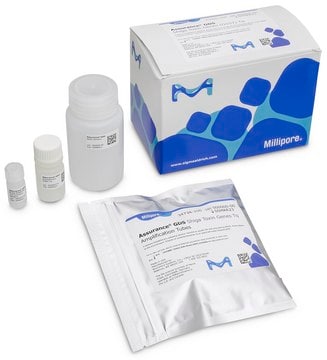
Components
Each 100 kit includes:
- Listeria monocytogenes Amplification Tubes Tq (0.2 mL)
- Listeria Concentration Reagent
- Listeria Resuspension Buffer Tq
- Wash Solution
Compatibility
BioControl GDS
Testing Method
PCR, Immunomagnetic Separation (IMS)
Legal Information
AOAC is a registered trademark of AOAC International
Assurance is a registered trademark of Merck KGaA, Darmstadt, Germany
Assurance GDS is a registered trademark of BioControl Systems, Inc.
PICKPEN is a registered trademark of Merck KGaA, Darmstadt, Germany
Rotor-Gene is a registered trademark of Qiagen GmbH
configured for
Product No.
Description
Pricing
required but not provided
Product No.
Description
Pricing
Storage Class
10 - Combustible liquids
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service