05164
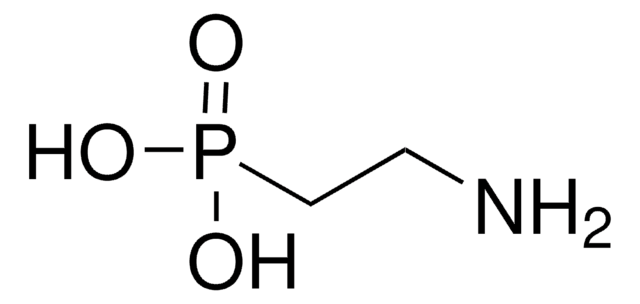
(Aminomethyl)phosphonic acid
PESTANAL®, analytical standard
Synonym(s):
AMPA
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
assay
≥98.0% (TLC)
shelf life
limited shelf life, expiry date on the label
impurities
≤3.0% water (calc. from elemental analysis)
application(s)
agriculture
environmental
format
neat
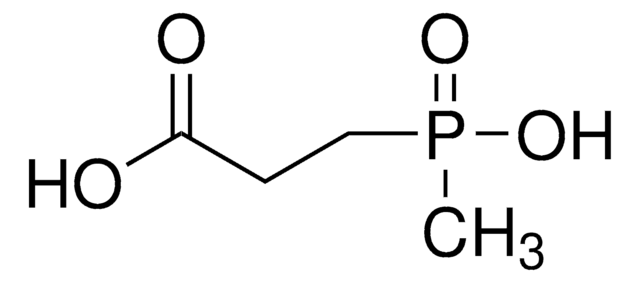
SMILES string
NCP(O)(O)=O
InChI
1S/CH6NO3P/c2-1-6(3,4)5/h1-2H2,(H2,3,4,5)
InChI key
MGRVRXRGTBOSHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Recommended products
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glyphosate and related compounds are measured in oatmeal and infant cereal using ion-exchange polymer-based particles for HPLC and SPE. Low level detection was obtained.
A simplified LC-MS/MS method to determine glyphosate and related compounds using a Supel™ Carbon LC U/HPLC column for stability under higher pH conditions and sufficient retention for the polar analytes in the presence of methanol as an extraction solvent.
Protocols
EPA Method 547 outlines the analysis of glyphosate in drinking water by direct aqueous injection HPLC, post column derivatization, and fluorescence detection
LC/MS Analysis of Glyphosate and Metabolites on apHera™ NH2, 2 mm I.D. Column
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service