8.52102
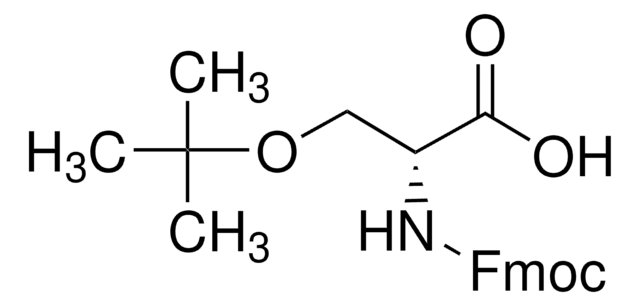
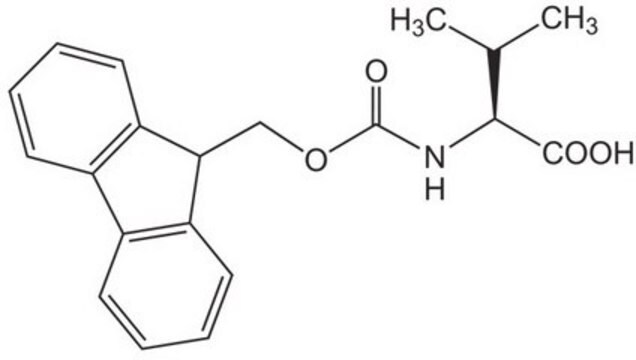
Fmoc-Glu(biotinyl-PEG)-OH
≥97% (TLC), for peptide synthesis, Novabiochem®
Synonym(s):
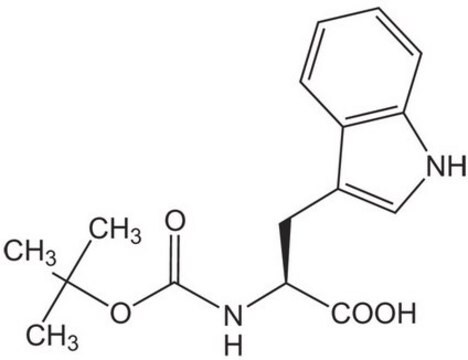
Fmoc-Glu(biotinyl-PEG)-OH, N-α-Fmoc-N-γ-(N-biotinyl-3-(2-(2-(3-aminopropyloxy)-ethoxy)-ethoxy)-propyl)-L-glutamine
About This Item
Recommended Products
product name
Fmoc-Glu(biotinyl-PEG)-OH, Novabiochem®
Quality Level
product line
Novabiochem®
assay
≥95.0% (HPLC)
≥97% (TLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
biotin
storage temp.
2-8°C
InChI
1S/C40H55N5O10S/c46-35(14-6-5-13-34-37-33(26-56-34)43-39(50)45-37)41-17-7-19-52-21-23-54-24-22-53-20-8-18-42-36(47)16-15-32(38(48)49)44-40(51)55-25-31-29-11-3-1-9-27(29)28-10-2-4-12-30(28)31/h1-4,9-12,31-34,37H,5-8,13-26H2,(H,41,46)(H,42,47)(H,44,51)(H,48,49)(H2,43,45,50)/t32-,33-,34-,37-/m0/s1
InChI key
MGOWNVYDCIBVKC-FNHRVDEZSA-N
Related Categories
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Biotinylation Reagents for Peptide Synthesis
Literature references
[1] B. Baumeister, et al. (2003) Biopolymers, 71, 339.
[2] X. Zhou et al., (2004) J. Am. Chem. Soc., 126, 15656.
[3] B. F. Gilmore, et al. (2006) Biochem. Biophys. Res. Commun,, 347, 373.
[4] C. T. Archer, et al. (2005) Mol. BioSyst., 1, 366.
Application
- Unbiased peptoid combinatorial cell screen identifies plectin protein as a potential biomarker for lung cancer stem cells: Utilized Fmoc-Glu(biotinyl-PEG)-OH in the synthesis protocol for peptoid libraries, contributing to biomarker discovery in lung cancer research (AC Raymond et al., 2019).
- Identification of side arm-modified DOTA scaffolds as multi-site binding ligands for cancer cells over normal cells: Included Fmoc-Glu(biotinyl-PEG)-OH in a synthesis protocol to enhance biotinylated scaffold properties for selective cancer cell targeting (V Rustagi, DG Udugamasooriya, 2019).
- TANGO-inspired design of anti-amyloid cyclic peptides: Employed Fmoc-Glu(biotinyl-PEG)-OH in the synthesis of cyclic peptides aimed at studying amyloid protein interactions, crucial for Alzheimer′s disease research (X Lu, RM Murphy, 2016).
- Converting a weaker ATP-binding site inhibitor into a potent hetero-bivalent ligand by tethering to a unique peptide sequence derived from the same kinase: Used Fmoc-Glu(biotinyl-PEG)-OH in developing new kinase inhibitors with improved binding properties (SR Kedika, DG Udugamasooriya, 2018).
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (TLC(CMA2)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (0,2 mmol in 1 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Biotin-labelled peptides have many important applications in immunology and histochemistry, such as affinity purification and FRET-based flow cytometry, solid-phase immunoassays, and receptor localization, that exploit the high affinity of streptavidin and avidin for biotin.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service