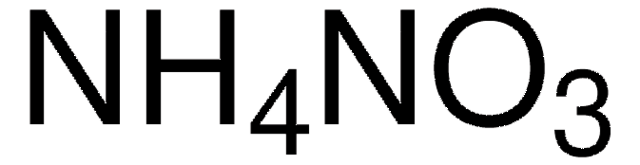1.01187
Ammonium nitrate
EMPLURA®
Synonym(s):
Ammonium nitrate, Nitric acid ammonia, Ammonia nitrate
About This Item
Recommended Products
Quality Level
product line
EMPLURA®
assay
≥95.0% (alkalimetric)
form
solid
potency
2462 mg/kg LD50, oral (Rat)
ign. residue
≤0.05% (as sulfate)
pH
4.5-7.0 (20 °C, 100 g/L in H2O)
bp
210 °C/15.0 hPa
mp
169 °C
solubility
1920 g/L
density
1.72 g/cm3 at 20 °C
bulk density
600‑700 kg/m3
anion traces
chloride (Cl-): ≤0.002%
phosphate (PO43-): ≤0.0005%
sulfate (SO42-): ≤0.01%
cation traces
Ca: ≤0.005%
Fe: ≤0.0005%
Mg: ≤0.001%
heavy metals (as Pb): ≤0.001%
storage temp.
2-30°C
InChI
1S/NO3.H3N/c2-1(3)4;/h;1H3/q-1;/p+1
InChI key
DVARTQFDIMZBAA-UHFFFAOYSA-O
Related Categories
Application
- Synthesis of some derivatives of 1,8-dioxo-octa-hydro xanthene and 9-aryl-hexahydro acridine-1,8-dione using metal ion-exchanged NaY zeolite as heterogeneous catalyst.: This paper discusses the synthesis of derivatives using metal ion-exchanged NaY zeolite as a catalyst. The study provides insights into ammonium nitrate′s role in catalytic processes (Niasar and Moradian, 2024).
- The Effect of the Solution Flow and Electrical Field on the Homogeneity of Large-Scale Electrodeposited ZnO Nanorods.: This research examines how solution flow and electrical fields affect the homogeneity of electrodeposited ZnO nanorods, highlighting the use of ammonium nitrate in the deposition process (Zhao et al., 2024).
- Development of an oxazole-based cleavable linker for peptides.: This research focuses on the development of an oxazole-based cleavable linker for peptides, utilizing ammonium nitrate in the synthesis process (Taggart et al., 2024).
Analysis Note
Identity: passes test
Chloride (Cl): ≤ 0.002 %
Phosphate (PO₄): ≤ 0.0005 %
Sulfate (SO₄): ≤ 0.01 %
Heavy metals (as Pb): ≤ 0.001 %
Ca (Calcium): ≤ 0.005 %
Fe (Iron): ≤ 0.0005 %
Mg (Magnesium): ≤ 0.001 %
Residue on ignition (as sulfate): ≤ 0.05 %
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 3
Storage Class
5.1C - Ammonium nitrate and ammonium nitrate containing preparations
wgk_germany
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service