C70908
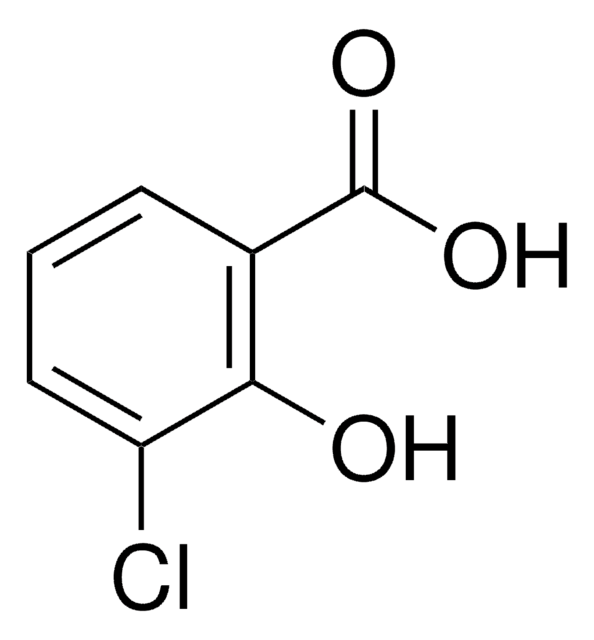
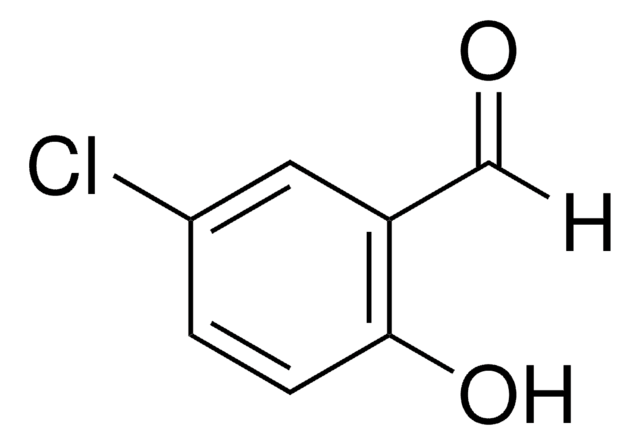
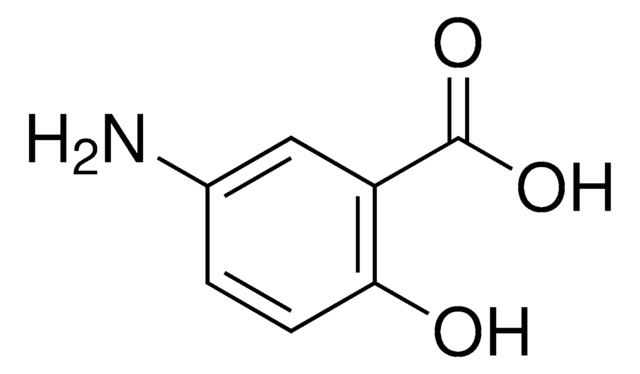
5-Chlorosalicylic acid
98%
Synonym(s):
5-Chloro-2-hydroxybenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
ClC6H3(OH)CO2H
CAS Number:
Molecular Weight:
172.57
Beilstein/REAXYS Number:
2046665
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
powder
mp
171-172 °C (lit.)
SMILES string
OC(=O)c1cc(Cl)ccc1O
InChI
1S/C7H5ClO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,(H,10,11)
InChI key
NKBASRXWGAGQDP-UHFFFAOYSA-N
Gene Information
human ... ALB(213)
Looking for similar products? Visit Product Comparison Guide
Related Categories
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M A Rubio et al.
Archives of microbiology, 145(2), 123-125 (1986-07-01)
The hybrid strain Pseudomonas sp. WR4016 was subcultivated with increasing concentrations of 5-chlorosalicylate (5----10 mM) as sole carbon source over a period of 9 months. At intervals of approximately 3 months derivative strains WR4017, WR4018 and WR4019 were isolated which
Comparative gastric ulcerogenic effects of meseclazone, 5-chlorosalicylic acid and other nonsteroidal anti-inflammatory drugs following acute and repeated oral administration to rats.
W Diamantis et al.
Toxicology and applied pharmacology, 52(3), 454-461 (1980-03-15)
Debarati Ray et al.
Physical chemistry chemical physics : PCCP, 14(35), 12182-12192 (2012-08-08)
The present work demonstrates the effect of biological confinement on the photophysics and dynamics of a bio-active drug molecule viz., 5-chlorosalicylic acid (5ClSA). 5ClSA is a potential candidate exhibiting Excited-State Intramolecular Proton Transfer (ESIPT) reaction and thereby generating the phototautomer
M A Rubio et al.
Archives of microbiology, 145(2), 116-122 (1986-07-01)
Methylsalicylate-grown cells of Pseudomonas sp. WR401 cometabolized 3-, 4- and 5-substituted halosalicylates to the corresponding halocatechols. Further degradation was unproductive due to the presence of high levels of catechol 2,3-dioxygenase. This strain acquired the ability to utilize 3-chlorobenzoate following acquisition
Hamdy A H Aly et al.
Applied and environmental microbiology, 74(12), 3812-3822 (2008-04-29)
Rhodococcus sp. strain HA01, isolated through its ability to utilize dibenzofuran (DBF) as the sole carbon and energy source, was also capable, albeit with low activity, of transforming dibenzo-p-dioxin (DD). This strain could also transform 3-chlorodibenzofuran (3CDBF), mainly by angular
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service