B60800
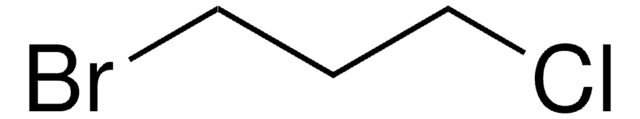
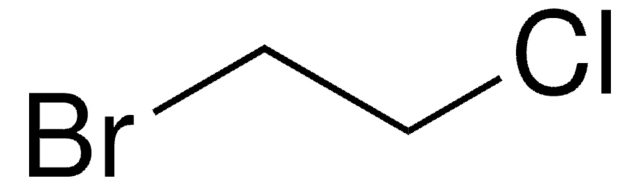
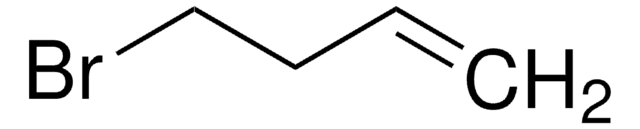
1-Bromo-4-chlorobutane
99%
Synonym(s):
Tetramethylene chlorobromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Cl(CH2)4Br
CAS Number:
Molecular Weight:
171.46
Beilstein/REAXYS Number:
1098275
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.4875 (lit.)
bp
80-82 °C/30 mmHg (lit.)
density
1.488 g/mL at 25 °C (lit.)
SMILES string
ClCCCCBr
InChI
1S/C4H8BrCl/c5-3-1-2-4-6/h1-4H2
InChI key
NIDSRGCVYOEDFW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
140.0 °F - closed cup
flash_point_c
60 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephen Verespy et al.
Scientific reports, 6, 24043-24043 (2016-04-08)
Allosteric partial inhibition of soluble, monomeric proteases can offer major regulatory advantages, but remains a concept on paper to date; although it has been routinely documented for receptors and oligomeric proteins. Thrombin, a key protease of the coagulation cascade, displays
Fulai Yang et al.
Scientific reports, 9(1), 18067-18067 (2019-12-04)
Camptothecin (CPT), a natural alkaloid isolated from Camptotheca acuminata Decne, is found to show potential insecticidal activities with unique action mechanisms by targeting at DNA-topoisomease I (Top1) complex and inducing cell apoptosis. To improve the efficacy against insect pests, two
Nan Li et al.
Toxicology in vitro : an international journal published in association with BIBRA, 41, 159-167 (2017-02-22)
The in vitro EpiSkin™ test method was validated in 2007 by the European Union Reference Laboratory for alternatives to animal testing (EURL ECVAM) as a full replacement method for the Draize acute skin irritation test and adopted in the OECD
Ngoc-Duc Doan et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(5), 387-391 (2014-11-18)
The solid-phase synthesis of azapeptides possessing a C-terminal aza-residue has been accomplished by a protocol featuring regioselective alkylation of benzhydrylidene-aza-glycinamide and illustrated by the syntheses of [aza-Lys(6)] growth-hormone-releasing peptide-6 analogs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service