ALD00564
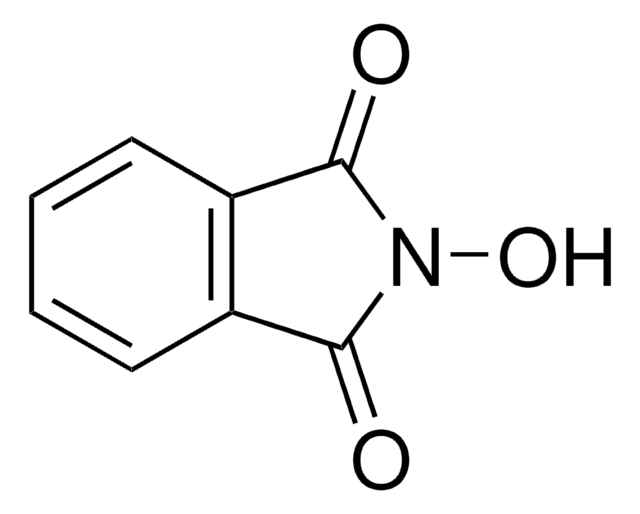
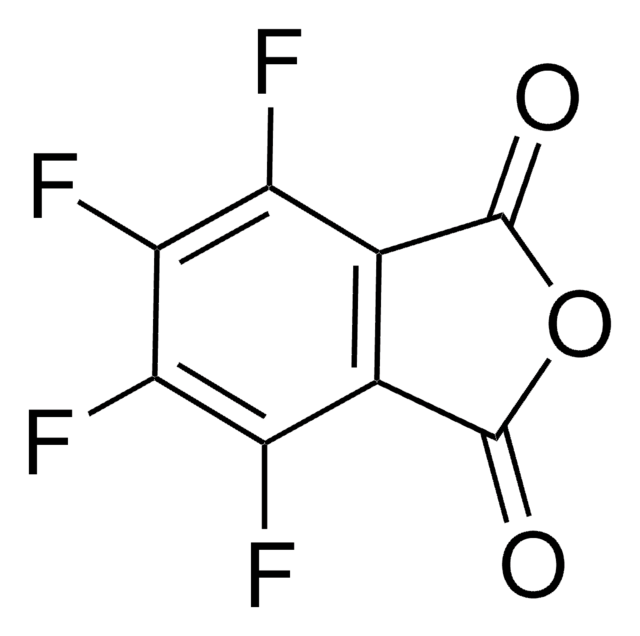
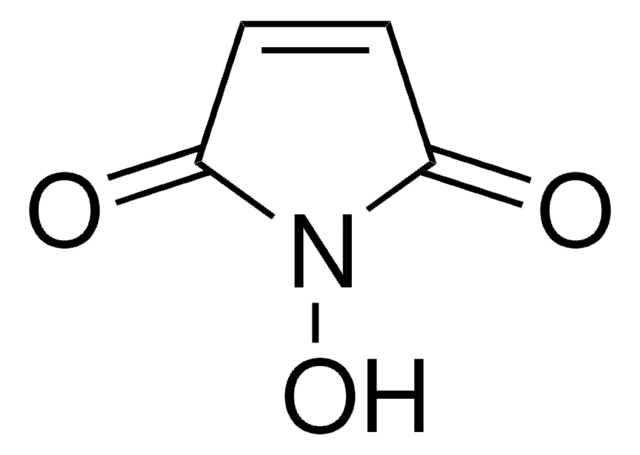
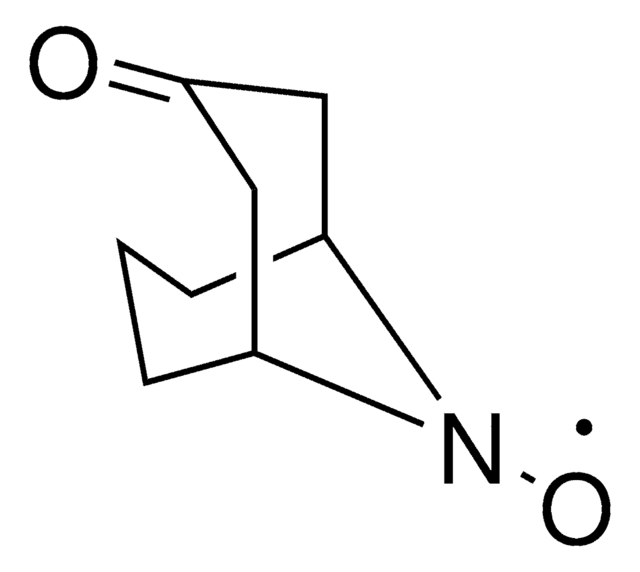
N-Hydroxytetrachlorophthalimide
Synonym(s):
4,5,6,7-Tetrachloro-2-hydroxy-1H-isoindole-1,3(2H)-dione, Tetrachloro-N-hydroxyphthalimide
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: oxidant
SMILES string
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI key
UTRBHXSKVVPTLY-UHFFFAOYSA-N
General description
Application
Other Notes
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Phil Baran and coworkers have developed a new reagent, N-Hydroxytetrachlorophthalimide (ALD00564), which provides a cheap, scalable, and safe synthetic alternative to highly used transformations like allylic oxidations, as well as Negishi and Suzuki–Miyaura type cross-coupling reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service