All Photos(1)
About This Item
Empirical Formula (Hill Notation):
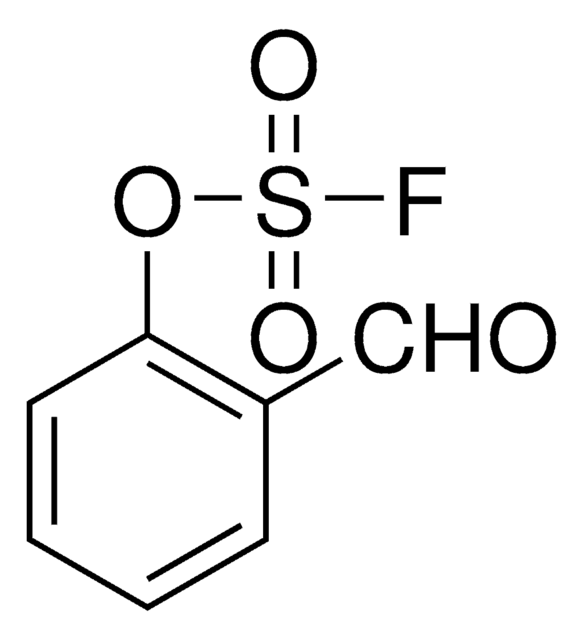
C7H4FNO3S
Molecular Weight:
201.17
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
form
liquid
Quality Level
SMILES string
O=S(F)(OC1=CC=CC=C1C#N)=O
InChI
1S/C7H4FNO3S/c8-13(10,11)12-7-4-2-1-3-6(7)5-9/h1-4H
InChI key
FWHISILUCPTNBH-UHFFFAOYSA-N
Related Categories
General description
2-Cyanophenyl sulfofluoridate is an aryl fluorosulfate. It undergoes Suzuki-Miyaura reaction with aryl boronic acid in the presence of Pd(OAc)2 and Et3N to afford the corresponding biaryl derivative. 2-Cyanophenyl sulfofluoridate can be synthesized from the reaction between phenol and sulfuryl fluoride in the presence of triethylamine.
Application
The following aryl fluorosulfate can be utilized as a cross-coupling partner in Suzuki-Miyaura reactions. The standard reaction conditions for these palladium-catalyzed C-C bond formations are ligand-free and can be performed in water, at room temperatre and are not air sensitive.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Qiaobin Liang et al.
Organic letters, 17(8), 1942-1945 (2015-04-10)
Aryl fluorosulfates were prepared by a simple method and employed as coupling partners in the Suzuki-Miyaura reaction. The cross-coupling reactions were performed in water under air at room temperature without ligands or additives such as surfactants or phase-transfer reagents and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service