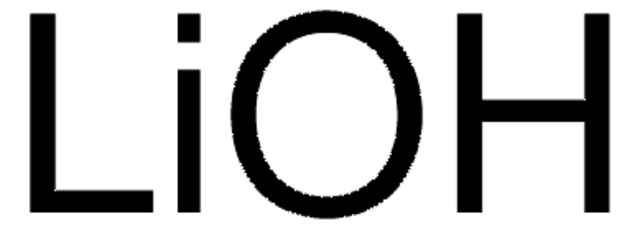
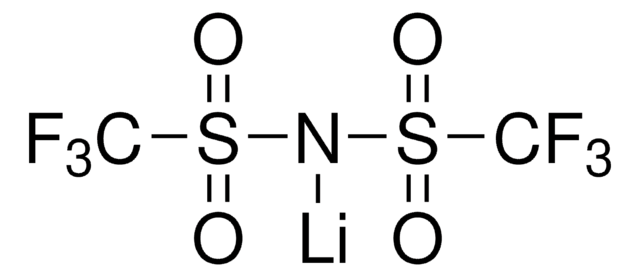
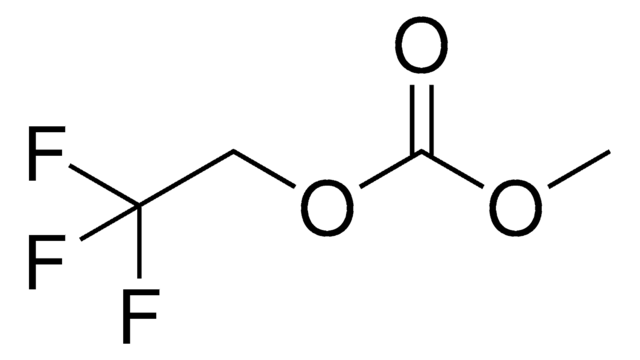
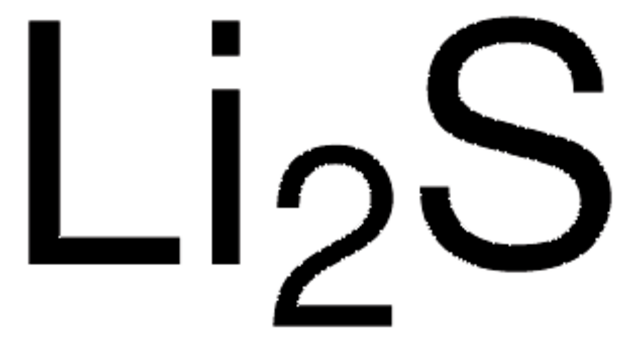930946
Lithium nitrate
battery grade, ≥99.9% trace metals basis
Synonym(s):
Lithium salt of nitric acid
About This Item
Recommended Products
Quality Level
grade
battery grade
assay
≥99.9% trace metals basis
form
powder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤0.5 wt. % H2O
≤1000 ppm (trace metals analysis)
mp
264 °C (lit.)
solubility
H2O: soluble (highly soluble(lit.))
acetone: soluble ((lit.))
alcohols: soluble ((lit.))
anion traces
chloride (Cl-): ≤500 ppm
sulfate (SO42-): ≤200 ppm
application(s)
battery manufacturing
greener alternative category
SMILES string
[Li+].[O-][N+]([O-])=O
InChI
1S/Li.NO3/c;2-1(3)4/q+1;-1
InChI key
IIPYXGDZVMZOAP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Lithium nitrate is produced by the acid-base reaction between nitric acid and lithium carbonate, which evolves carbon dioxide and water. The resulting material is dried, purified, and heated to form the anhydrous product.
Application
Because lithium nitrate is soluble in water, researchers also use lithium nitrate in the synthesis of lithium compounds using a host of solution-based chemistries. For example, microwave-induced combustion using solutions of lithium nitrate has yielded olivine-type lithium iron phosphate (LiFePO4), lithium cobalt oxide (LiCoO2), and lithium titanium oxides (ex. Li4Ti5O12 and Li2TiO3). Hydrothermal processing, sol-gel processing, spray pyrolysis, co-precipitation pre-processing, and Li emulsion-drying methods have all used lithium nitrate as a reactant to form lithium metal oxides. These techniques can yield controlled particle size, grain size, crystallinity, or facilitate the introduction of dopants for engineering the properties of the products, often explored for next-generation lithium-ion batteries.
Our battery grade lithium nitrate with ≥99.9% trace metals purity and low chloride and sulfate impurities, is designed as a precursor for cathode materials for lithium-ion batteries.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 3
Storage Class
5.1B - Oxidizing hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service