929484
FBnG-C3-PEG1-C3-NH2 hydrochloride
≥95%
Synonym(s):
(R)-2-acetamido-3-((2-amino-9-(4-fluorobenzyl)-6-oxo-6,9-dihydro-1H-purin-8-yl)thio)-N-(3-(3-aminopropoxy)propyl)propanamide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C23H31FN8O4S · xHCl
Molecular Weight:
534.61 (free base basis)
UNSPSC Code:
12352101
NACRES:
NA.21
Recommended Products
Quality Level
assay
≥95%
form
powder
functional group
amine
storage temp.
2-8°C
SMILES string
O=C1NC(N)=NC2=C1N=C(SC[C@@H](C(NCCCOCCCN)=O)NC(C)=O)N2CC3=CC=C(C=C3)F.Cl
Related Categories
Application
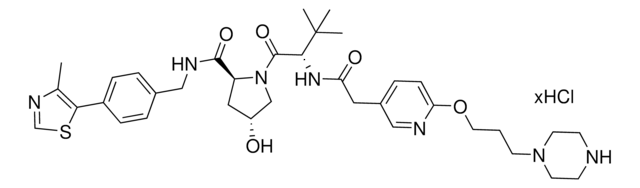
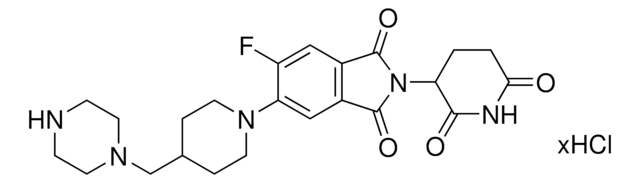
Protein degrader building block FBnG-C3-PEG1-C3-NH2 hydrochloride enables the synthesis of molecules for degradation of proteins and PROTAC® (proteolysis-targeting chimeras) research. This conjugate contains a p-fluorobenzylguanine (FBnG) ligand, a PEG linker, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and degrader, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate degrader libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Other Notes
Legal Information
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service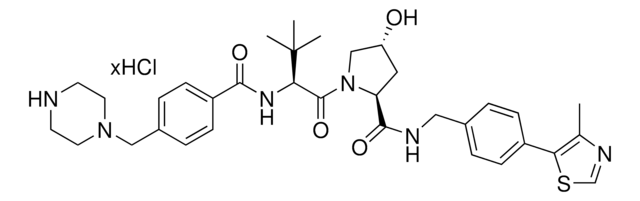
![Poly[(9,9-dioctyl-2,7-divinylenefluorenylene)-alt-{2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene}]](/deepweb/assets/sigmaaldrich/product/structures/303/311/48d96c0b-9463-4dd8-9ccf-3a67b7c781c5/640/48d96c0b-9463-4dd8-9ccf-3a67b7c781c5.png)

![1,4-Bis[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene ≥95%](/deepweb/assets/sigmaaldrich/product/structures/348/602/d338a771-03b4-4ba9-9788-426be85146d9/640/d338a771-03b4-4ba9-9788-426be85146d9.png)