914304
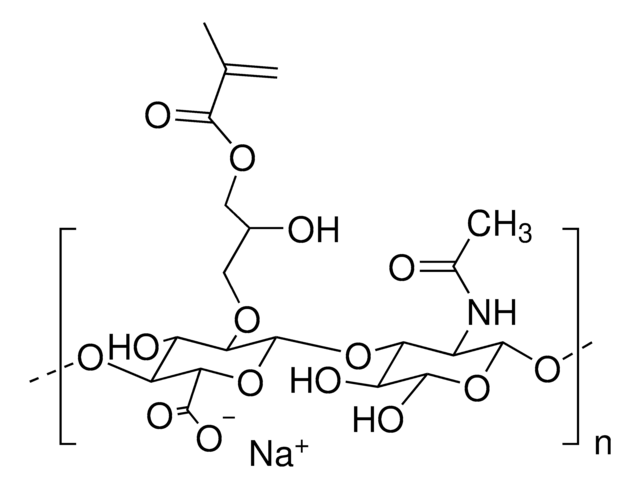
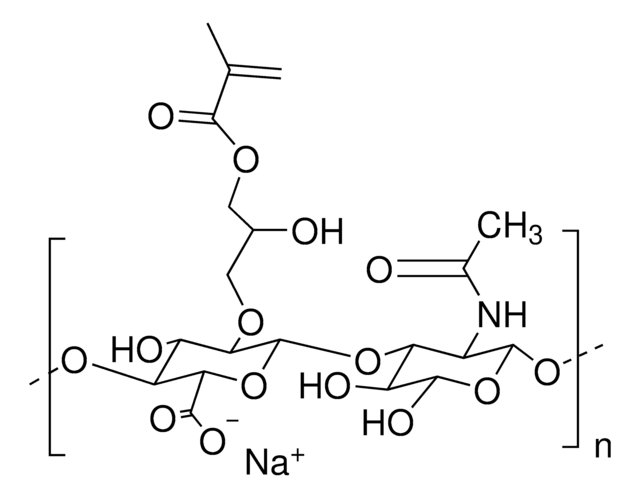
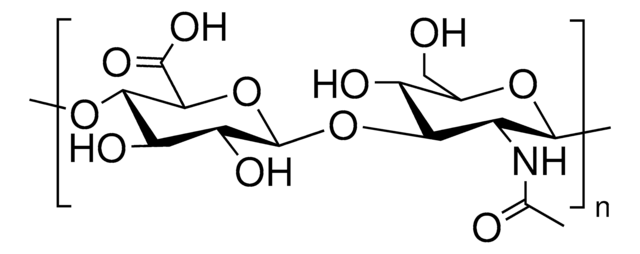
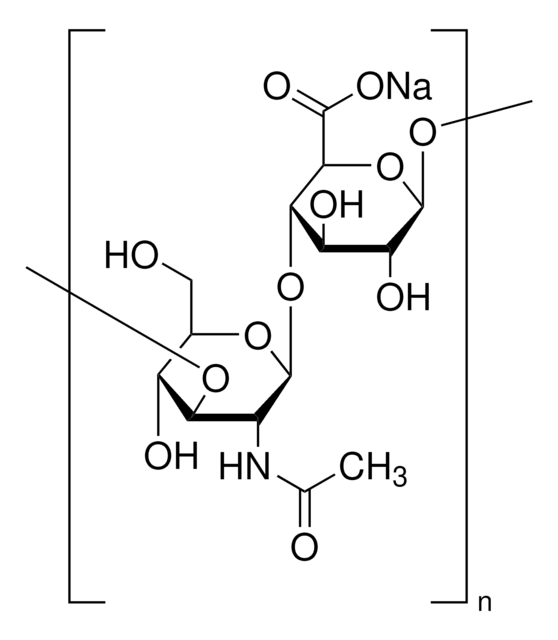
Hyaluronic acid methacrylate
average degree of substitution 35%, average Mw 175000
Synonym(s):
Functioanlized hyaluronic, HA methacrylamide, HAMA, Hyaluronic acid MA
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(NaC20H28NO15)n
UNSPSC Code:
12352106
NACRES:
NA.23
form:
(powder or chunk(s) or fibers)
Recommended Products
description
NMR: Conforms to structure
Quality Level
form
(powder or chunk(s) or fibers)
mol wt
average Mw 175000
color
white to off-white
storage temp.
2-8°C
Related Categories
Application
Hyaluronic acid (HA) is a linear polysaccharide of alternating D-glucuronic acid and N-acetyl-D-glucosamine found primarily in connective tissues. HA based hydrogels are widely used in tissue engineering, 3D bioprinting, and drug deliery applications. The methacrylate functionalized hyaluronic acid is photo-crosslinkable, and can be used to generate crosslinked hydrogels.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cindy Chung et al.
Tissue engineering. Part A, 15(2), 243-254 (2009-02-06)
Mesenchymal stem cells (MSCs) are multipotent progenitor cells whose plasticity and self-renewal capacity have generated significant interest for applications in tissue engineering. The objective of this study was to investigate MSC chondrogenesis in photo-cross-linked hyaluronic acid (HA) hydrogels. Because HA
Judy Yeh et al.
Biomaterials, 27(31), 5391-5398 (2006-07-11)
Encapsulation of mammalian cells within hydrogels has great utility for a variety of applications ranging from tissue engineering to cell-based assays. In this work, we present a technique to encapsulate live cells in three-dimensional (3D) microscale hydrogels (microgels) of controlled
Aleksander Skardal et al.
Tissue engineering. Part A, 16(8), 2675-2685 (2010-04-15)
Bioprinting by the codeposition of cells and biomaterials is constrained by the availability of printable materials. Herein we describe a novel macromonomer, a new two-step photocrosslinking strategy, and the use of a simple rapid prototyping system to print a proof-of-concept
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service