All Photos(2)
About This Item
Empirical Formula (Hill Notation):
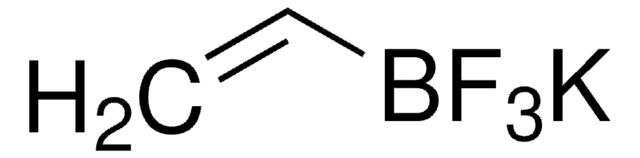
C8H7BF3KO2
CAS Number:
Molecular Weight:
242.04
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
SMILES string
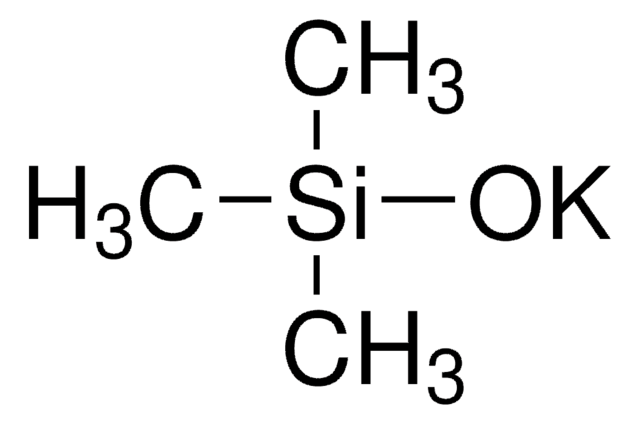
O=C(B(F)(F)F)C1=CC=C(OC)C=C1.[K]
InChI
1S/C8H7BF3O2.K.H/c1-14-7-4-2-6(3-5-7)8(13)9(10,11)12;;/h2-5H,1H3;;
InChI key
VXVQBCXLJYVTDZ-UHFFFAOYSA-N
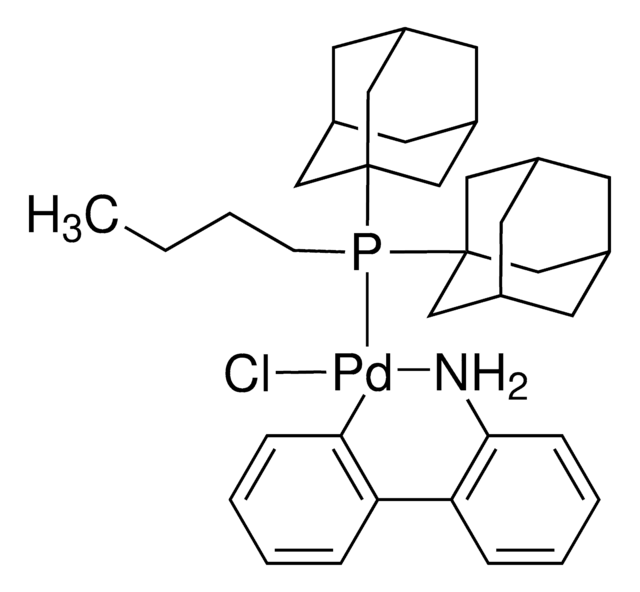
Application
Acyltrifluroborates (KAT′s) are bench, air, and moisture stable reagents for rapid, chemoselective amide bond formations with hydroxylamines. These amide bond forming reactions proceed under aqueous conditions, without the need for coupling reagents or protecting groups. This product was introduced in collaboration with the Bode Research Group.
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service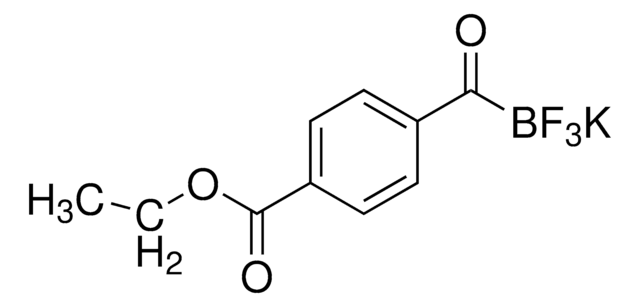
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)