771953
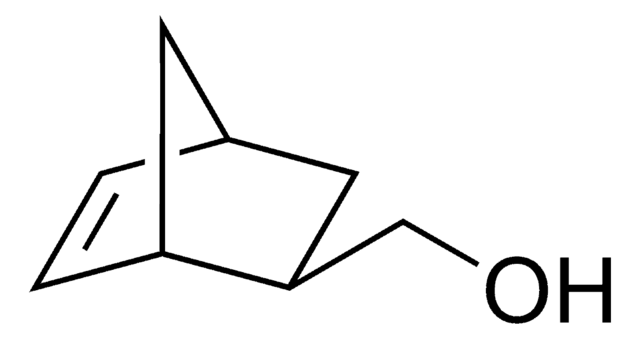
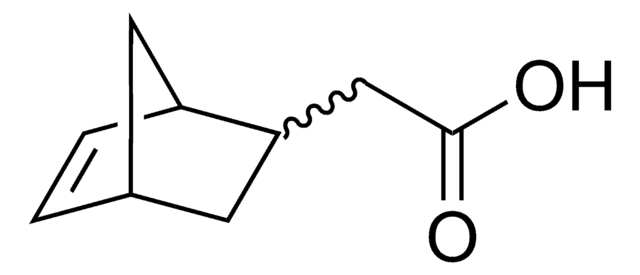
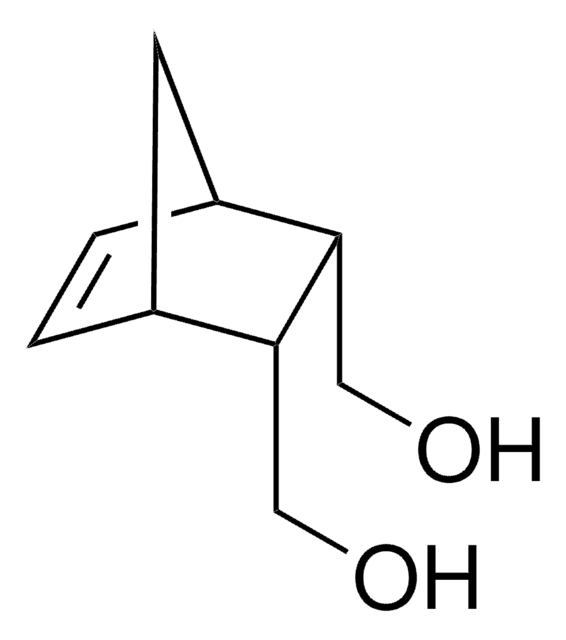
exo-5-Norbornene-2-methanol
≥99% (exo)
Synonym(s):
exo-5-Norbornenyl methyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H12O
CAS Number:
Molecular Weight:
124.18
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
assay
≥99% (exo)
form
liquid
refractive index
n20/D 1.499
bp
200-205/760 mmHg
density
1.030 at 25 °C
SMILES string
OCC1C[C@@H]2C[C@H]1C=C2
InChI
1S/C8H12O/c9-5-8-4-6-1-2-7(8)3-6/h1-2,6-9H,3-5H2/t6-,7+,8?/m0/s1
InChI key
LUMNWCHHXDUKFI-KJFJCRTCSA-N
General description
Exo-5-Norbornene-2-methanol is a norbornene derivative which is widely used as a monomer in the synthesis of polynorbornenes by vinyl polymerisation or ring opening methathesis polymerisation (ROMP). They are also used as a monomer or an intermediate in the synthesis of photoresist materials. This material is also widely used as an intermediate in the synthesis of pharmaceutically and biologically active compounds.
Application
Used as a monomer in the synthesis of polynorbornenes by ring opening metathesis polymerisation.
Features and Benefits
- Produces polymers with high thermal stability and transparency which are useful for optical applications.
Related product
Product No.
Description
Pricing
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Water-soluble polymers from acid-functionalized norbornenes
Lienkamp, K., Kins, C. F., Alfred, S. F., Madkour, A. E., & Tew, G. N.
Journal of Polymer Science Part A: Polymer Chemistry, 47(5), 1266-1273 (2009)
Stereo-Selective Synthesis of 5-Norbornene-2-exo-carboxylic Acid-Rapid Isomerization and Kinetically Selective Hydrolysis.
Kanao, M., Otake, A., Tsuchiya, K., & Ogino, K.
International Journal of Organic Chemistry, 2, 26-30 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service