771406
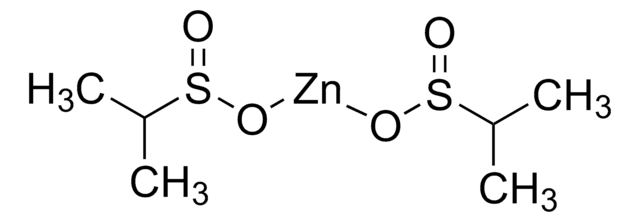
Zinc trifluoromethanesulfinate
Synonym(s):
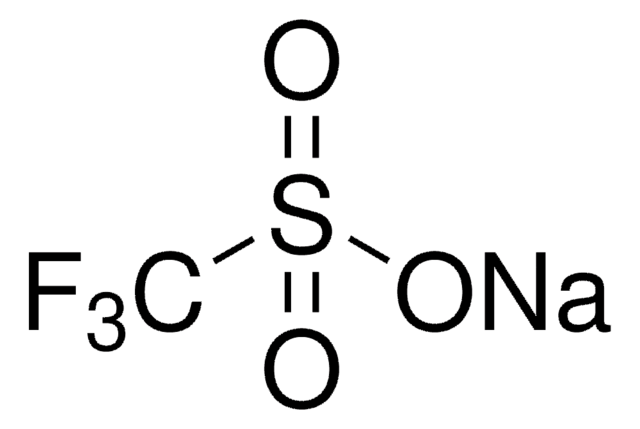
1,1,1-Trifluoro-methanesulfinic acid zinc salt (2:1), Baran trifluoromethylation reagent, Bis(((trifluoromethyl)sulfinyl)oxy)zinc, TFMS
About This Item
Recommended Products
form
solid
Quality Level
reaction suitability
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
mp
151-157 °C
storage temp.
2-8°C
SMILES string
FC(F)(F)S(=O)O[Zn]OS(=O)C(F)(F)F
Looking for similar products? Visit Product Comparison Guide
Application
Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
Linkage
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)