All Photos(2)
About This Item
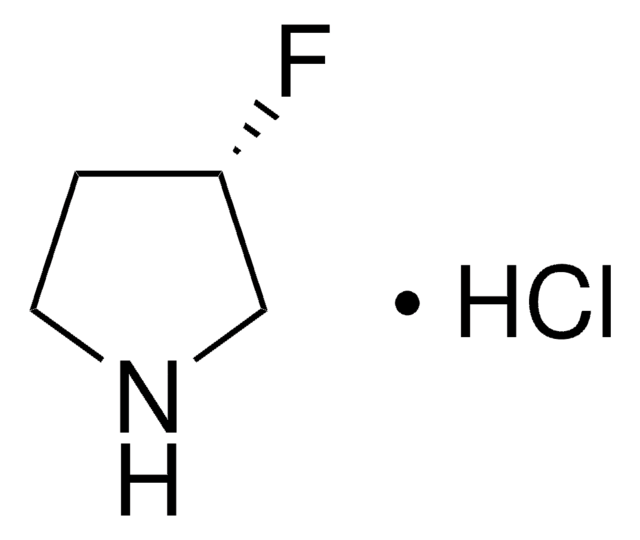
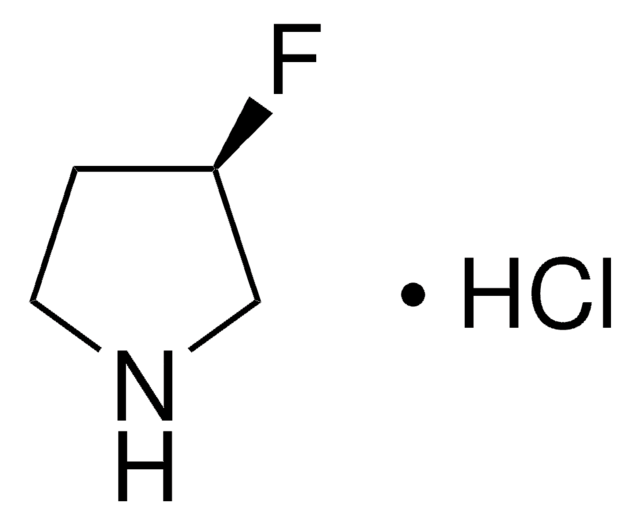
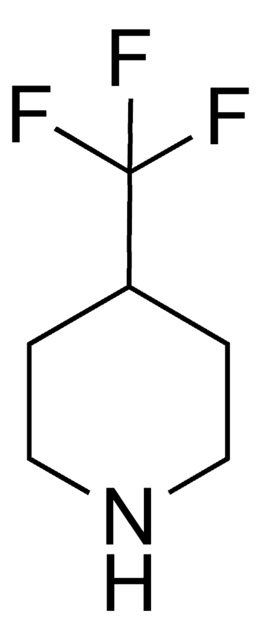
Empirical Formula (Hill Notation):
C4H7F2N · HCl
CAS Number:
Molecular Weight:
143.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
133-136 °C
functional group
fluoro
SMILES string
Cl.FC1(F)CCNC1
InChI
1S/C4H7F2N.ClH/c5-4(6)1-2-7-3-4;/h7H,1-3H2;1H
InChI key
YYVPZQADFREIFR-UHFFFAOYSA-N
Application
3,3-Difluoropyrrolidine hydrochloride can be used as a building block in the synthesis of:
It can be also used as a reactant in the preparation of cyclic and acyclic β-aminofluoroalkenes via allylic amination using the Pd catalyst.
- Triazole substituted prolyl difluoropyrrolidines as potential inhibitors of dipeptidyl peptidase-4.
- Dual leucine zipper kinase (DLK) inhibitors.
It can be also used as a reactant in the preparation of cyclic and acyclic β-aminofluoroalkenes via allylic amination using the Pd catalyst.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, Synthesis, Structure-Activity Relationships, and Docking Studies of 1-(?-1, 2, 3-Triazol Substituted Prolyl)-(S)-3, 3-Difluoropyrrolidines as a Novel Series of Potent and Selective Dipeptidyl Peptidase-4 Inhibitors
Zhang L, et al.
Chemical Biology & Drug Design, 81(2), 198-207 (2013)
Activation of Allylic C-F bonds: Palladium-Catalyzed Allylic Amination of 3, 3-Difluoropropenes
Pigeon X, et al.
Angewandte Chemie (International Edition in English), 49(6), 1123-1127 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service