All Photos(3)
About This Item
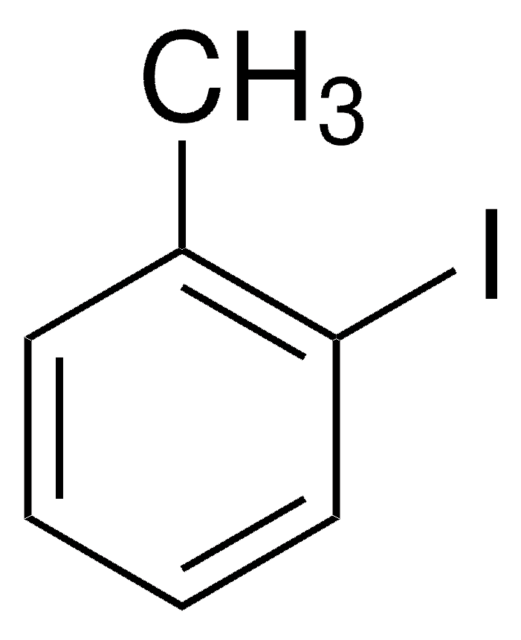
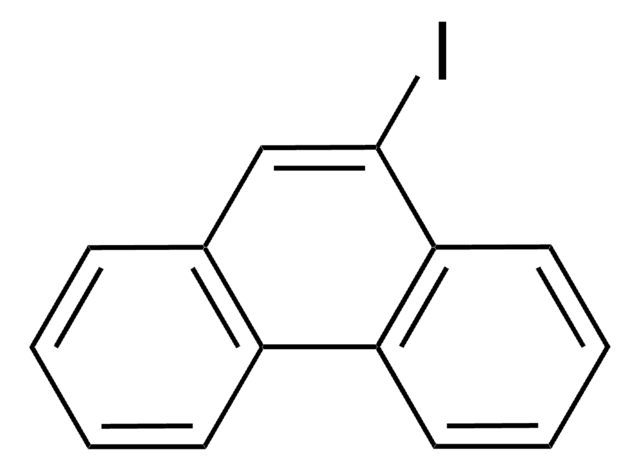
Empirical Formula (Hill Notation):
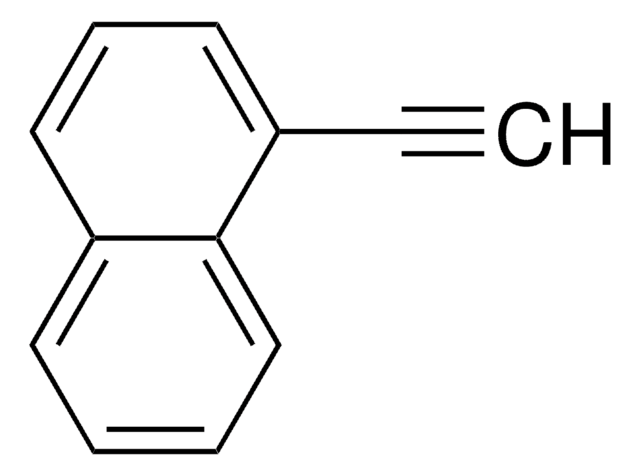
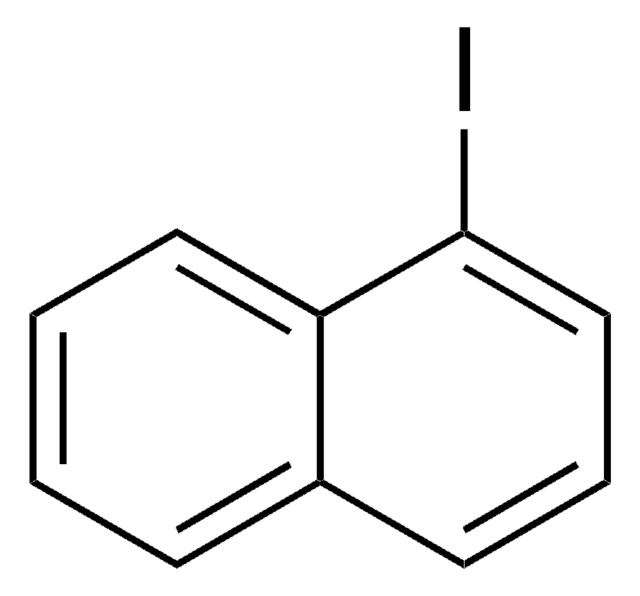
C10H7I
CAS Number:
Molecular Weight:
254.07
Beilstein/REAXYS Number:
1905951
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥99.0% (HPLC)
form
chunks
mp
52-56 °C
functional group
iodo
SMILES string
Ic1ccc2ccccc2c1
InChI
1S/C10H7I/c11-10-6-5-8-3-1-2-4-9(8)7-10/h1-7H
InChI key
FRNLBIWVMVNNAZ-UHFFFAOYSA-N
Related Categories
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Long Zhao et al.
Physical chemistry chemical physics : PCCP, 21(30), 16737-16750 (2019-07-20)
The three-ring polycyclic aromatic hydrocarbons (PAHs) 3H-benz[e]indene (C13H10) and 1H-benz[f]indene (C13H10) along with their naphthalene-based isomers 2-(prop-2-yn-1-yl)naphthalene (C13H10), 2-(prop-1-yn-1-yl)naphthalene (C13H10), and 2-(propa-1,2-dien-1-yl)naphthalene (C13H10) were formed through a "directed synthesis"via a high temperature chemical micro reactor under combustion-like conditions (1300 ±
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![5-iodo-2,3-dihydrobenzo[b]furan AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/397/941/052e6fc6-247f-4798-8183-78a5248eb1e7/640/052e6fc6-247f-4798-8183-78a5248eb1e7.png)