677396
Magnesium aluminate, spinel
nanopowder, <50 nm particle size (BET)
Synonym(s):
Magnesium aluminate
About This Item
Recommended Products
form
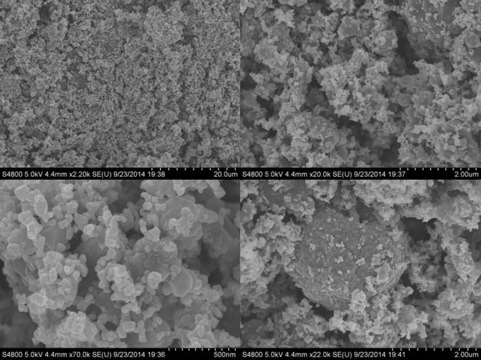
nanopowder
reaction suitability
reagent type: catalyst
core: magnesium
surface area
>30 m2/g
particle size
<50 nm (BET)
mp
2130 °C (lit.)
density
3.64 g/mL at 25 °C (lit.)
SMILES string
[Mg++].[O-][Al]=O.[O-][Al]=O
InChI
1S/2Al.Mg.4O/q;;+2;;;2*-1
InChI key
VPBIQXABTCDMAU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- A Review on Processing Polycrystalline Magnesium Aluminate Spinel (MgAl2O4): This review discusses various sintering techniques, material properties, and machinability of polycrystalline magnesium aluminate spinel, highlighting its optical transparency and mechanical properties (Z Shi et al., 2020).
- Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption: This study examines how the structure and composition of magnesium aluminate spinel affect its water adsorption capabilities, focusing on the interaction with gases at different spinel compositions (Y Mordekovitz et al., 2020).
- Spectroscopic Study of Ordering in Non-Stoichiometric Magnesium Aluminate Spinel: The paper explores the ordering within non-stoichiometric magnesium aluminate spinel and its impact on material properties (V Erukhimovitch et al., 2015).
- Transparent Polycrystalline Magnesium Aluminate Spinel Fabricated by Spark Plasma Sintering: This research presents the fabrication of transparent polycrystalline magnesium aluminate spinel using spark plasma sintering, analyzing its mechanical and optical properties (M Sokol et al., 2018).
- A Comparative Study on the Effect of Different Additives on the Formation and Densification of Magnesium Aluminate Spinel: Investigates the effects of various additives on the formation and densification of magnesium aluminate spinel, providing insights into the synthesis and optimization of this ceramic material (SK Mohan et al., 2016).
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service