569763
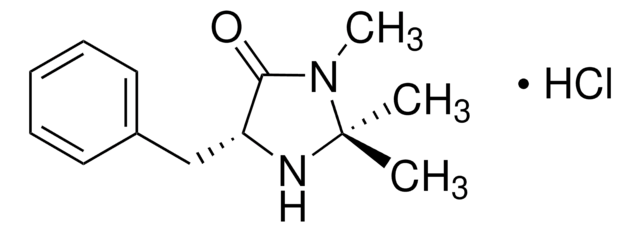
(5S)-(−)-2,2,3-Trimethyl-5-benzyl-4-imidazolidinone monohydrochloride
97%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
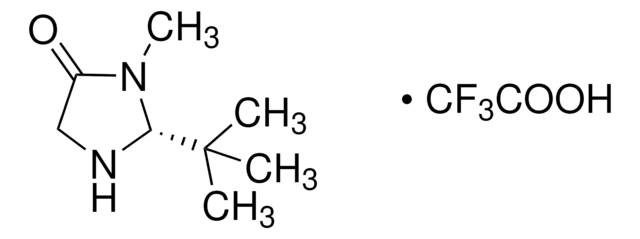
C13H18N2O · HCl
CAS Number:
Molecular Weight:
254.76
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
optical activity
[α]20/D -67°, c = 1 in H2O
mp
157-161 °C (lit.)
functional group
phenyl
SMILES string
Cl.CN1C(=O)[C@H](Cc2ccccc2)NC1(C)C
InChI
1S/C13H18N2O.ClH/c1-13(2)14-11(12(16)15(13)3)9-10-7-5-4-6-8-10;/h4-8,11,14H,9H2,1-3H3;1H/t11-;/m0./s1
InChI key
YIYFEXGDFJLJGM-MERQFXBCSA-N
Application
Metal-free OrganoCatalyst technology for asymmetric catalysis. Catalyzes asymmetric Diels-Alder reactions, 1,3-dipolar additions and pyrrole alkylations in high enantiomeric excess.
Used in first highly enantioselective organocatalytic Diels-Alder reaction and 1,3-dipolar addition.
Legal Information
U.S. Pat. 6,369,243 and related patents apply. For research purposes only.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ahrendt, K. A. et al
Journal of the American Chemical Society, 122, 4243-4243 (2000)
New strategies in organic catalysis: the first enantioselective organocatalytic Friedel-Crafts alkylation.
N A Paras et al.
Journal of the American Chemical Society, 123(18), 4370-4371 (2001-07-18)
Jen, W. S. et al
Journal of the American Chemical Society, 122, 9874-9874 (2000)
Articles
In collaboration with Materia, Inc., we are pleased to offer six imidazolidinone OrganoCatalysts™.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service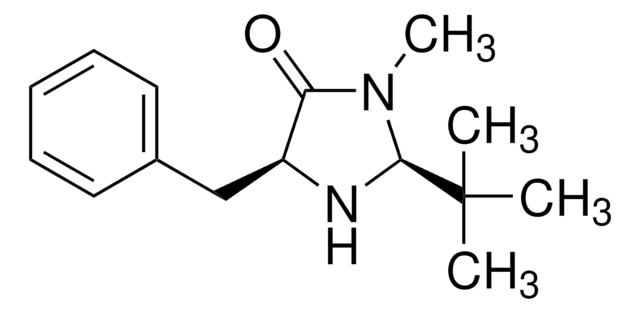


![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)