All Photos(1)
About This Item
Empirical Formula (Hill Notation):
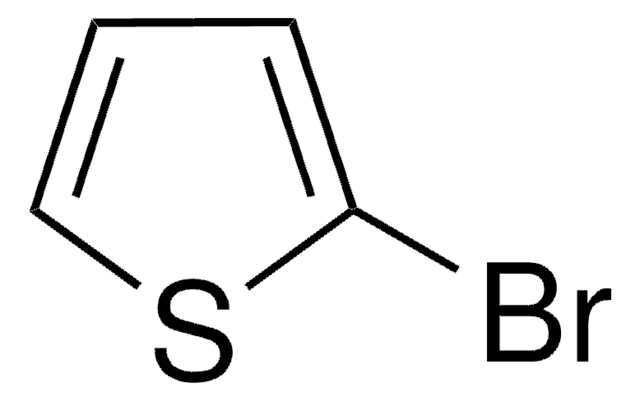
C8H5BrS2
CAS Number:
Molecular Weight:
245.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
96%
form
solid
mp
29-32 °C (lit.)
SMILES string
Brc1ccc(s1)-c2cccs2
InChI
1S/C8H5BrS2/c9-8-4-3-7(11-8)6-2-1-5-10-6/h1-5H
InChI key
OMOAIGVIYUXYAU-UHFFFAOYSA-N
General description
5-Bromo-2,2′-bithiophene is a bromothiophene derivative. Its reaction with various aryl iodides bearing an electron-donating or electron-withdrawing substituent has been described. It can be synthesized from 2,2′-bithiophene.
Application
5-Bromo-2,2′-bithiophene may be used in the synthesis of trimethyl-[2,2′;5′,2″;5″,2″]quaterthiophen-5-yl-silane (4TTMS) and 5-hexylsulfanyl-2,2′:5′,2′′-terthiophene.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mark E Roberts et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12134-12139 (2008-08-20)
The development of low-cost, reliable sensors will rely on devices capable of converting an analyte binding event to an easily read electrical signal. Organic thin-film transistors (OTFTs) are ideal for inexpensive, single-use chemical or biological sensors because of their compatibility
Kei Kobayashi et al.
Organic letters, 7(22), 5083-5085 (2005-10-21)
[reaction: see text] Bromothiophene derivatives react with aryl iodides catalyzed by a palladium complex in the presence of a silver(I) nitrate/potassium fluoride system to induce coupling at the C-H bond, while the carbon-bromine bond is intact. The produced coupling product
Silole-Containing. pi.-Conjugated Systems. 3.1 A Series of Silole-Thiophene Cooligomers and Copolymers: Synthesis, Properties, and Electronic Structures.
Tamao K, et al.
Macromolecules, 28(25), 8668-8675 (1995)
Three-dimensional tetra (oligothienyl) silanes as donor material for organic solar cells.
Roquet S, et al.
Journal of Materials Chemistry, 16(29), 3040-3045 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service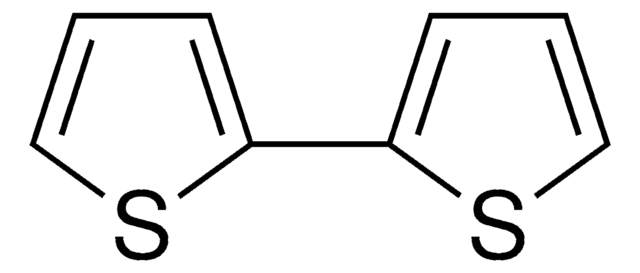

![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)