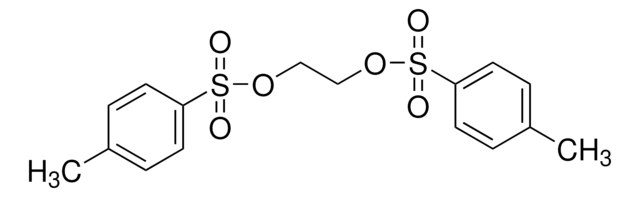
518352
3,4-Dihydro-2H-pyran-2-methanol, polymer-bound
extent of labeling: ~0.7 mmol/g loading, 1 % cross-linked
Synonym(s):
DHP resin, Ellman′s DHP resin
About This Item
Recommended Products
crosslinking
1 % cross-linked
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
reactivity: alcohol reactive
extent of labeling
~0.7 mmol/g loading
SMILES string
C=Cc1ccccc1.C=Cc2ccc(C=C)cc2.C3CC(COCc4ccccc4)OC=C3
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Ellman group has participated in the development of a variety of C-H functionalization methods. An electron rich phosphine ligand has proven to be very useful for a variety of Rh(I)-catalyzed C-C bond forming reactions applicable to heterocycle synthesis as exemplified in the recent Science paper “Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis.” Another useful ligand developed for the highly functional group compatible direct arylation of nitrogen heterocycles is described in a 2008 J. Am. Chem. Soc. paper “Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope through Mechanistic Insight.” The Ellman group also developed the chiral amine reagent tert-Butanesulfinamide, which is extensively used in academics and industry for the asymmetric synthesis of amines. A comprehensive survey of tert-Butanesulfinamide methods and applications up through 2009 is provided in the 2010 Chemical Reviews article, “Synthesis and Applications of tert-Butanesulfinamide.”
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service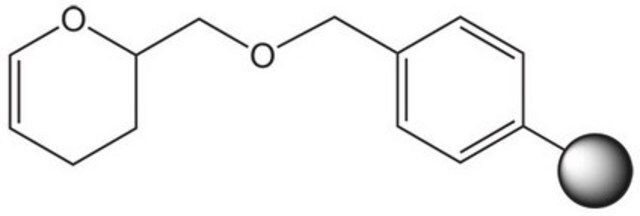
![2-{2-[2-(2-Mercaptoethoxy)ethoxy]ethoxy}ethanol 97%](/deepweb/assets/sigmaaldrich/product/structures/130/969/7d2ec2b4-e0f1-4836-aeb2-139994173612/640/7d2ec2b4-e0f1-4836-aeb2-139994173612.png)

![1,4-Bis[4-(11-acryloyloxyundecyloxy)benzoyloxy]-2-methylbenzene](/deepweb/assets/sigmaaldrich/product/structures/215/829/62728d6f-7668-4693-94e2-37abc69d1144/640/62728d6f-7668-4693-94e2-37abc69d1144.png)