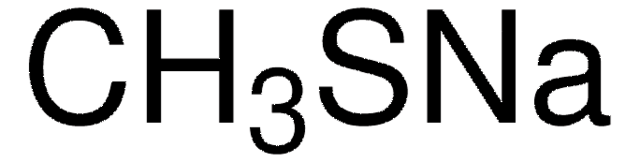516686
Sodium thiomethoxide solution
21% in H2O
Synonym(s):
Sodium methanethiolate solution, Sodium methanethiolate, Sodium thiomethoxide solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
CH3NaS
Molecular Weight:
70.09
Beilstein/REAXYS Number:
3592983
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
21% in H2O
Looking for similar products? Visit Product Comparison Guide
Related Categories
Legal Information
Product of Arkema
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
80.6 °F - closed cup
flash_point_c
27 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Peter C Tyler et al.
Journal of the American Chemical Society, 129(21), 6872-6879 (2007-05-10)
Transition state theory suggests that enzymatic rate acceleration (kcat/knon) is related to the stabilization of the transition state for a given reaction. Chemically stable analogues of a transition state complex are predicted to convert catalytic energy into binding energy. Because
Mild, selective deprotection of thioacetates using sodium thiomethoxide.
Wallace OB and Springer DM.
Tetrahedron Letters, 39(18), 2693-2694 (1998)
Surface-initiated reversible addition-fragmentation chain transfer (RAFT) polymerization from fine particles functionalized with trithiocarbonates.
Ohno K, et al.
Macromolecules, 44(22), 8944-8953 (2011)
Y P Pang et al.
FEBS letters, 502(3), 93-97 (2001-10-05)
Using the computer docking program EUDOC, in silico screening of a chemical database for inhibitors of human adenovirus cysteine proteinase (hAVCP) identified 2,4,5,7-tetranitro-9-fluorenone that selectively and irreversibly inhibits hAVCP in a two-step reaction: reversible binding (Ki = 3.09 microM) followed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service