513830
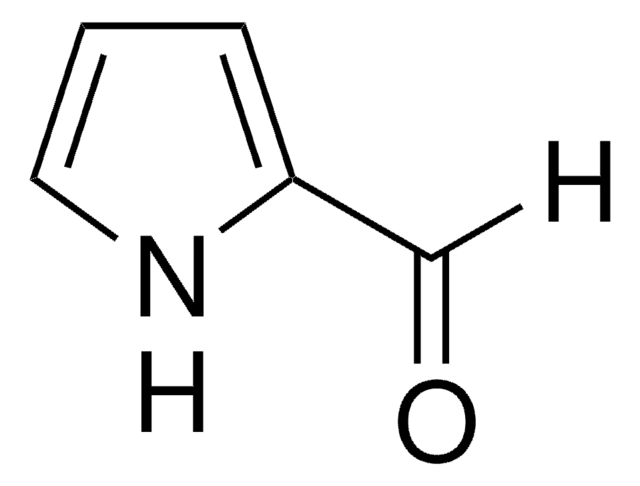
Indole-5-carboxaldehyde
98%
Synonym(s):
5-Formylindole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
100-103 °C (lit.)
functional group
aldehyde
SMILES string
O=Cc1ccc2[nH]ccc2c1
InChI
1S/C9H7NO/c11-6-7-1-2-9-8(5-7)3-4-10-9/h1-6,10H
InChI key
ADZUEEUKBYCSEY-UHFFFAOYSA-N
Related Categories
Application
Indole-5-carboxaldehyde can be used as a reactant in the:
- Preparation of curcumin derivatives as anti-proliferative & anti-inflammatory agents
- Preparation of analogs of botulinum neurotoxin serotype A protease inhibitors
- Stereoselective synthesis of dibenzylideneacetone derivatives as β-amyloid imaging probes
- Synthesis of para-para stilbenophanes by McMurry coupling
- Stereoselective synthesis of heteroaromatic (E)-α,β-unsaturated ketones from aldehydes
- Structure-based drug design of aurora kinase A inhibitors
- Preparation of 5-indolyl linked 15- and 18-membered azacrown ethers to study their cation-π interactions.
- Preparation of bibenzimidazole derivatives substituted 5-indolyl moiety in the study of inhibition of topoisomerase I activity.
- Synthesis of (5-(4-(3,4,5-trimethoxybenzoyl)-1H-imidazol-2-yl)-1H-indol-2-yl)(3,4,5-trimethoxyphenyl)methanone and radioiodinated indolochalcone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Raquel Álvarez et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(12), 3406-3419 (2011-02-24)
The synthesis of a new family of methoxy-substituted [2.7]- and [2.8]paracyclophanes linked by 3-oxapentamethylene-1,5-dioxy and hexamethylene-1,6-dioxy bridges has been carried out by using the McMurry methodology. Related indole compounds were also synthesised. Olefin-to-diol ratios depended on the bridge length, the
Balducci, E.; et al.
European Journal of Organic Chemistry, 311-311 (2011)
Houlihan WJ.
The Chemistry of Heterocyclic Compounds, 367-367 (2009)
Mohane Selvaraj Coumar et al.
Journal of medicinal chemistry, 52(4), 1050-1062 (2009-01-15)
Aurora kinases have emerged as attractive targets for the design of anticancer drugs. Through structure-based virtual screening, novel pyrazole hit 8a was identified as Aurora kinase A inhibitor (IC(50) = 15.1 microM). X-ray cocrystal structure of 8a in complex with
Petr Capek et al.
ACS chemical neuroscience, 2(6), 288-293 (2011-07-12)
Botulinum neurotoxin (BoNT), the etiological agent that causes the neuroparalytic disease botulism, has become a highly studied drug target in light of the potential abuse of this toxin as a weapon of bioterrorism. In particular, small molecule inhibitors of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service