512338
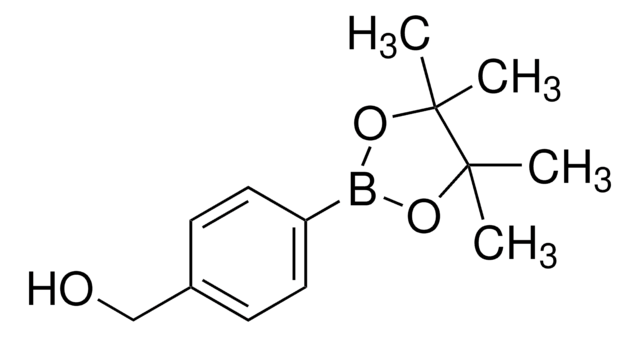
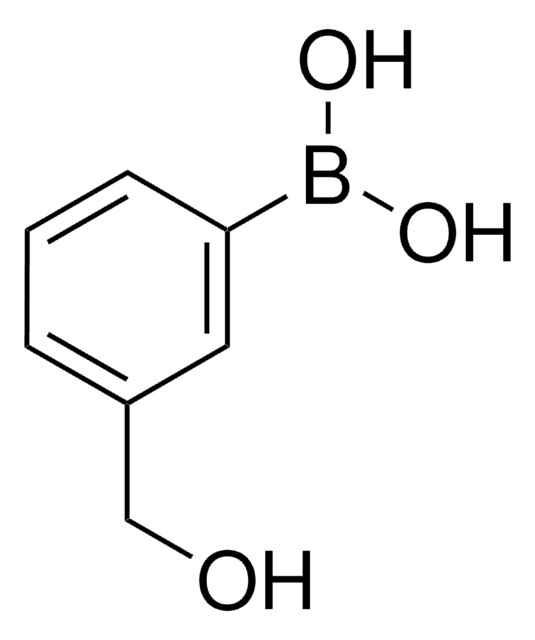
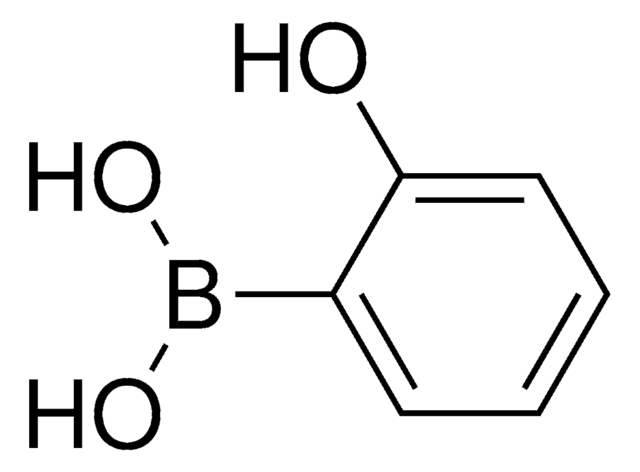
4-(Hydroxymethyl)phenylboronic acid
≥95%
Synonym(s):
α-Hydroxy-p-tolueneboronic acid, 4-Hydroxymethylbenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2C6H4B(OH)2
CAS Number:
Molecular Weight:
151.96
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥95%
mp
251-256 °C (lit.)
functional group
hydroxyl
SMILES string
OCc1ccc(cc1)B(O)O
InChI
1S/C7H9BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,9-11H,5H2
InChI key
PZRPBPMLSSNFOM-UHFFFAOYSA-N
Application
Reactant involved in the synthesis of biologically active compounds including:
Reactant involved in:
Involved in the synthesis of polyurethane containing spindle-type chromophores
- Imidazo[4,5-b]pyrazin-2-ones for use as mTOR kinase inhibitors
- Human immunodificiency virus protease inhibitors with activity against resistant viruses
Reactant involved in:
- Suzuki coupling reactions
- Copper-catalyzed transformation from arylboronic acids in water
Involved in the synthesis of polyurethane containing spindle-type chromophores
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Asymmetry, 14, 3435-3446 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service