480835
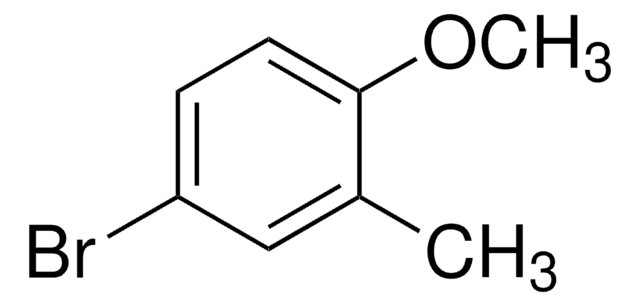
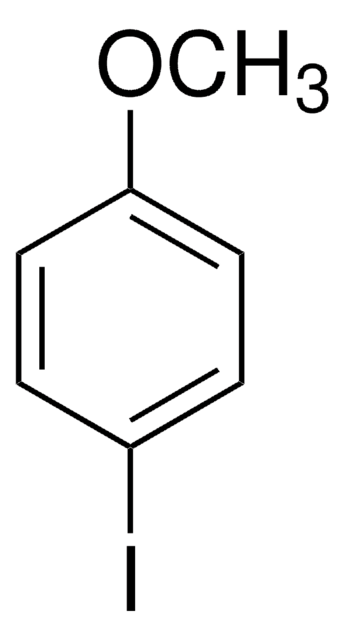
2-Bromo-4-methylanisole
97%
Synonym(s):
3-Bromo-4-methoxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH3)C6H3OCH3
CAS Number:
Molecular Weight:
201.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.565 (lit.)
bp
124-125 °C/20 mmHg (lit.)
mp
15.5 °C (lit.)
density
1.392 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
COc1ccc(C)cc1Br
InChI
1S/C8H9BrO/c1-6-3-4-8(10-2)7(9)5-6/h3-5H,1-2H3
InChI key
DHPUIKWBNXTXOB-UHFFFAOYSA-N
General description
2-Bromo-4-methylanisole can be prepared via bromination of 4-methylanisole using poly(4-vinylpyridinium bromochromate).
Application
2-Bromo-4-methylanisole may be used in the synthesis of:
- 1,6-bis(2-hydroxy-5-methylphenyl)pyridine (H2mdppy)
- 1,8-dimethoxy-4-methylanthra-quinone
- ethyl 2-(2-methoxy-5-methylphenyl)-2-methyl-4-oxocyclopentanecarboxylate
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Unambiguous synthesis and spectral characterization of 1,8-dihydroxy-4-methylanthraquinone.
Wang A, et al.
ARKIVOC (Gainesville, FL, United States), 1, 80-84 (2002)
Poly (4-vinylpyridinium bromochromate): an efficient reagent for bromination of aromatic compounds.
Albadi J, et al.
Monatshefte fur Chemie / Chemical Monthly, 144(2), 179-181 (2013)
Lipase-Promoted Access to Phenolic Herbertane-Type Sesquiterpenes: (+)-1, 14-Herbertenediol, (-)-a-Herbertenol, (-)-Herbertenediol and Their Enantiomers.
Acherar S, et al.
European Journal of Organic Chemistry, 2004(24), 5092-5099 (2004)
Highly efficient white organic electroluminescence from a double-layer device based on a boron hydroxyphenylpyridine complex.
Liu Y, et al.
Angewandte Chemie (International Edition in English), 41(1), 182-184 (2002)
Total synthesis of (?)-herbertenolide by stereospecific formation of vicinal quaternary centers in a crystalline ketone.
Ng D, et al.
Organic Letters, 6(4), 645-647 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service